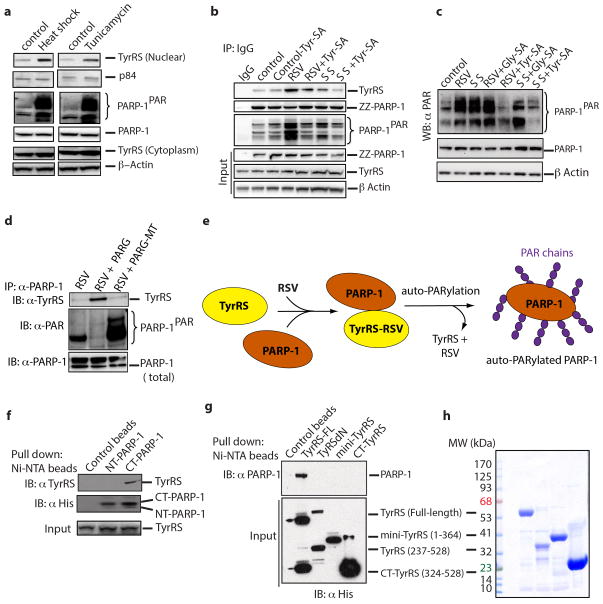
Extended Data Figure 3. Resveratrol facilitates the TyrRS/PARP-1 interaction in an active-site-dependent manner.
a, Both heat shock (42°C for 30 min) and tunicamycin-treatment (10 μg/ml, ER stress) facilitated the nuclear translocation of TyrRS and activation of PARP-1. b, Resveratrol or serum starvation facilitate TyrRS interaction with PARP-1 and Tyr-SA prevents this interaction. ZZ-PARP-1 was immunoprecipiated with IgG from HeLa cells treated with RSV or serum starvation alone or in combination with Tyr-SA. c, Resveratrol or serum starvation mediated PARP-1 activation is blocked only by Tyr-SA and not by Gly-SA. d, TyrRS interacts directly with PARP-1. HeLa cell lysate after RSV treatment (5 μM, 30 min) was divided into three parts and treated with PARG and catalytically inactive PARG-MT. PARP-1 was immunoprecipitated and analyzed for TyrRS interaction. e. Model illustrating the mechanism of RSV mediated TyrRS interaction with PARP-1 and subsequent release after auto-PARylation. f. Ni-NTA pull-down of N- and C-terminal fragments of PARP-1 overexpressed in E. coli demonstrated that TyrRS interacts with the C-terminal region of PARP-1. g, Only the full-length TyrRS (1-528) and none of the various fragments of TyrRS (mini-TyrRS (1-364), ΔN-TyrRS (228-528) or the C-domain (328-528)) interacts with PARP-1. h, Coomassie blue staining of a gel showing the total protein input in for the experiment of Extended Data Figure 3g.