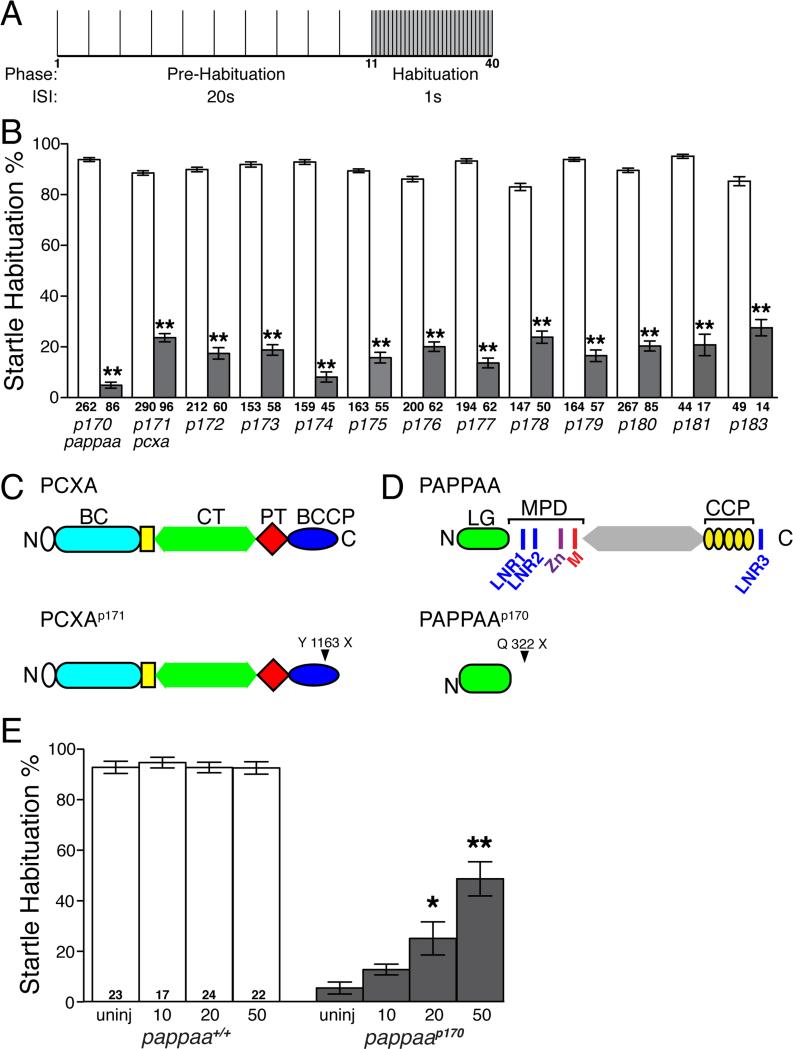
Figure 1. Genetic screen identifies mutations affecting acoustic startle habituation.
(A) Schematic of acoustic startle habituation assay. Larvae are exposed to 10 non-habituating acoustic stimuli, delivered at 20s interstimulus intervals (ISI), and then 30 habituating stimuli at a 1s ISI. (B) Mean acoustic startle habituation percentage calculated by comparing the average frequency of startle responsiveness of an individual to stimuli 1-10 and stimuli 31-40 (Wolman et al., 2011). Behaviorally defined wild-type siblings shown in white bars, mutants in grey bars. (C) Estimated truncated PCXAp171 protein in information overloadp171 mutants due to Y1163X mutation. BC: biotin carboxylase; CT: carboxyl transferase; PT: pyruvate carboxylase tetramerization; BCCP: biotincarboxy carrier protein. (D) Estimated truncated PAPP-AAp170 protein product in unfilteredp170 mutants due to Q322X mutation. LG: laminin G-like module; LNR: Lin-12/Notch repeats, MPD: metzincin proteolytic domain containing zinc-binding consensus sequence (Zn) and Met-turn motif (M), CCP: complement control protein modules 1-5. E) pappaap170 larvae injected with increasing doses of wild type pappaa mRNA show improved habituation at 5 dpf. *p<0.01, **p<0.001, ANOVA with Bonferonni correction versus wild-type sibling (B) or uninjected pappaap170 (E) larvae. N= number of larvae shown within or below each bar. Error bars indicate SEM.