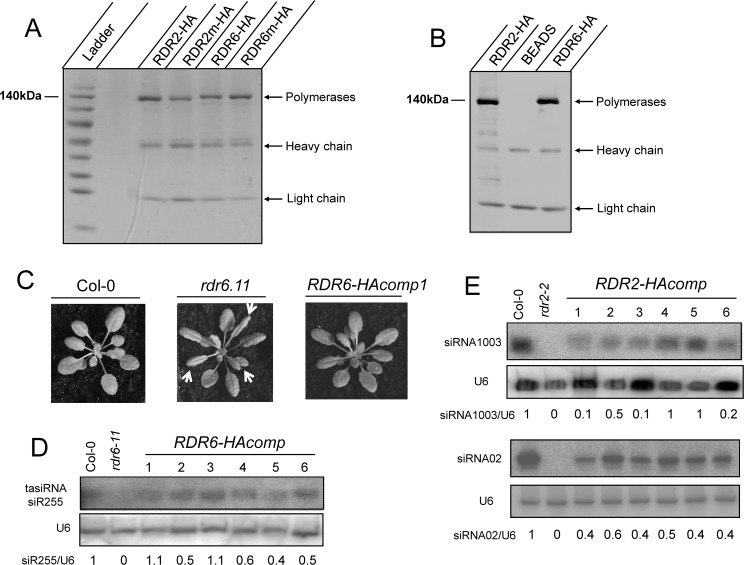
Fig 1. Purification of RDR-HA and RDRm-HA and complementation of rdr2–2 and rdr6–11 mutations by RDR2-HA and RDR6-HA.
(A) Coomassie blue stained protein gel of immunopurified RDRs. After immunoprecipitation, 2.5μl of HA-conjugated beads, corresponding to 250ng of proteins, were loaded on a 4–20% polyacrylamide gradient-SDS gel. Heavy and light chains from HA antibody are visible. (B) Western blot performed with anti-HA antibodies. After immunoprecipitation, 1μl of HA-conjugated beads were loaded on a 4–20% polyacrylamide gradient-SDS gel. The secondary antibody cross reacts with the heavy and light chains of the HA antibody. (C) 24-day-old Columbia wild type (Col-0) plant, rdr6–11 mutant and one rdr6–11 transgenic line containing the 35S::RDR6-HA transgene (RDR6-HAcomp1). The downward curling rosette leaf phenotype of rdr6–11 mutants (arrows) was rescued by RDR6-HA expression. (D) RNA blot analysis of the accumulation of the TAS1-derived tasiRNA siR255. The analysis was performed on rdr6–11 mutant and six independent 35S::RDR6-HA rdr6–11 transgenic lines. (E) RNA blot analysis of the accumulation of the heterochromatic siRNA1003 and siRNA02. The analysis was performed on rdr2–2 mutant and six independent 35S::RDR2-HA rdr2–2 transgenic lines (RDR2-HAcomp). U6 snRNA hybridization served as control for total RNA loading and normalized values with Col-0 control set to 1 are indicated.