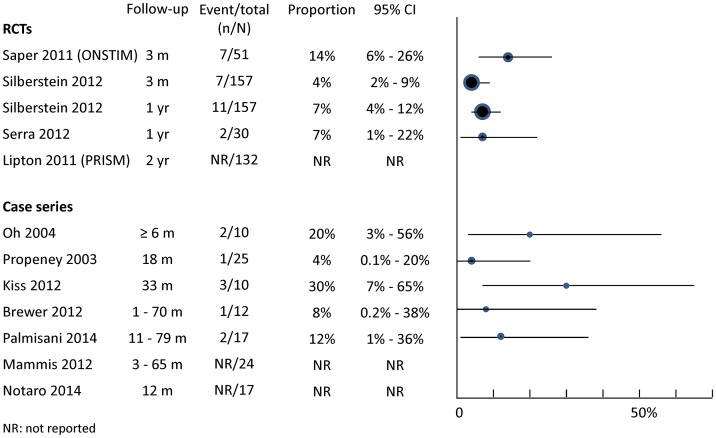
Fig 5. Adverse effects associated with implantation and/or use of occipital nerve stimulation: infections.
Saper 2011—the number shown was infections at site for lead/extension tract. There were additionally four ‘complications at incision sites’.[17] Silberstein et al. 2012—there were additionally ‘wound site complications’ (four at 3 months;[28] five at 1 year[30]). Lipton et al. described infections being the most frequent device-related adverse events but did not report the numbers in their published abstract.[16] The three cases described by Kiss and colleagues were ‘inflammation at surgical sites’ (3/10, 30%) that were treated with intravenous and oral antibiotics.[33] They stated that ‘neither blood nor wound cultures identified bacterial growth’.