Abstract
Rituximab is used for treatment of multiple haematological cancers. Caution for use is advised in patients with significant cardiorespiratory disease due to known cases of exacerbations of angina and arrhythmias. However, its cardiotoxicity profile is not as well recognised as other monoclonal antibodies such as transtuzumab. We report a case of a 66-year-old man who developed Takotsubo's cardiomyopathy (TC) after an elective infusion of rituximab. This case is exceptional in that rituximab has not been linked to TC, and the vast majority of chemotherapy-linked and immunotherapy-linked TC reactions have occurred during initial infusions. We also discuss the different mechanisms which link TC to immunotherapy and chemotherapy, and propose that there may be a potential for risk-stratifying recipients of this frequently used immunotherapy prior to administering treatment.
Background
Rituximab, a chimeric monoclonal anti-CD20 antibody, is used for the treatment of multiple haematological cancers. These include follicular non-Hodgkin's lymphoma and chronic lymphocytic leukaemia (CLL), in combination with chemotherapy. It is also being used off-label in selected autoimmune diseases such as rheumatoid arthritis and as antirejection treatment in organ transplants. Caution for use is advised in patients with a history of cardiorespiratory disease because exacerbations of angina, arrhythmias and heart failure have been reported.
There have been published cases linking chemotherapeutic agents such as 5-fluorouracil (5-FU) to Takotsubo's cardiomyopathy1 (TC). TC, also known as apical ballooning syndrome, is a non-ischaemic cardiomyopathy characterised by an acute stunning in the myocardium. It is often linked to physical and emotional stress and is most frequently reported in postmenopausal women. It is characterised by precordial ST-segment elevation, apical ballooning on echocardiography with a pronounced left ventricular (LV) dysfunction and normal or unobstructed coronary arteries on angiography.2–5 However, the incidence of chemotherapy-induced and immunotherapy-induced TC are very rare.
We describe the case of a 66-year-old man who developed TC after receiving a rituximab infusion for CLL, having received rituximab on multiple occasions in the past. To the best of our knowledge, this case is exceptional in that rituximab has not been linked to TC, and the vast majority of chemotherapy-linked TC reactions have occurred during initial infusions.
Case presentation
A 66-year-old man attended the outpatient department to receive a single-agent rituximab infusion. He had been diagnosed with stage C CLL in 2008, and had completed six cycles of fludarabine, cyclophosphamide and rituximab (FCR) chemotherapy. He experienced a relapse in February 2012, and received a further four cycles of FCR. He had further disease progression in 2013, in association with Epstein-Barr virus reactivation, and received pulsed methylprednisolone and rituximab starting in August 2013.
The patient also had a history of idiopathic thrombocytopaenic purpura, treated with steroids and intravenous immunoglobulins, immune agranulocytosis treated with steroids and cyclosporine, and insulin-dependent diabetes mellitus. He did not have any previous cardiac history.
Within 40 min of the infusion, the patient developed acute shortness of breath, facial flushing, rigours and spikes of temperature. The infusion was discontinued, and intravenous hydrocortisone and piriton were administered to little effect. He subsequently became tachycardic with a heart rate of 150 bpm and blood pressure of 100/62 mm Hg, doubly incontinent and began vomiting. Physical examination was unremarkable with no evidence of heart failure.
Investigations
A 12-lead ECG (figure 1) showed a sinus tachycardia of 130 bpm, associated with ST segment elevation in leads I, II and V4–V6. A troponin level was sent at the time of the event and returned showing 147 ng/L (normal range 0–40). An urgent transthoracic bedside echocardiogram was reported as showing normal LV size with hypokinesis of the anterior wall. There was evidence of mild mitral regurgitation, aortic regurgitation and pericardial effusion, but no signs of tamponade.
Figure 1.
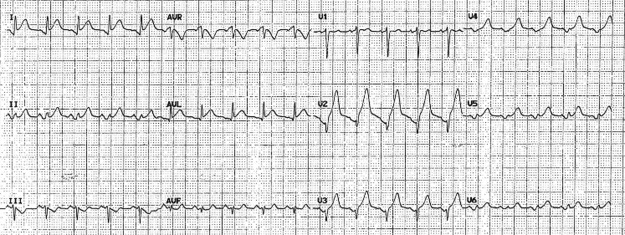
ECG tracing during acute episode.
A provisional diagnosis of an anterolateral ST-elevation myocardial infarction was made. Aspirin was administered and the patient was transferred urgently to a cardiothoracic centre for primary coronary intervention.
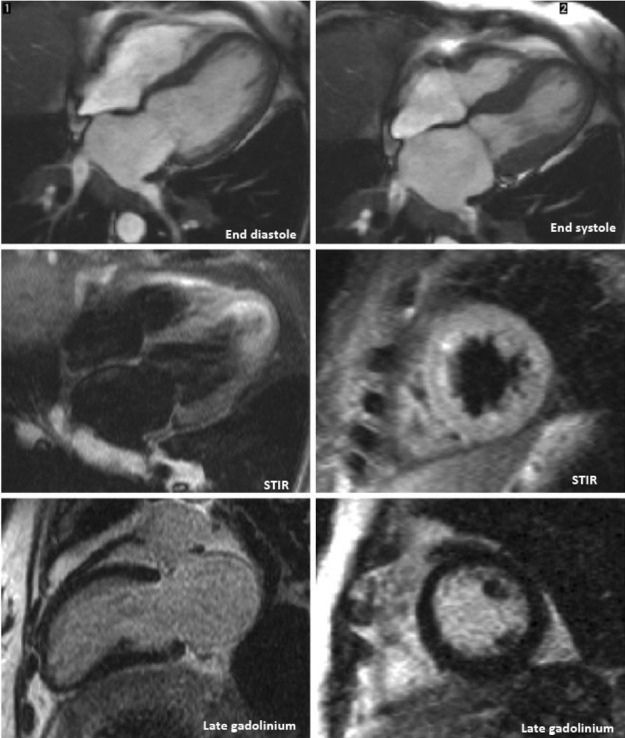
An urgent angiogram demonstrated non-obstructive distal atheroma with no indication for percutaneous intervention. The left ventriculogram from this episode is demonstrated in figure 2A, B. A repeat transthoracic echocardiogram revealed normal sized but severely hypokinetic/akinetic mid-apical, anterior and lateral walls. The estimated ejection fraction was 40% and the left atrium was dilated. There was evidence of mild mitral regurgitation and the right ventricle was normal. A subsequent cardiac MRI (figure 3) reported an akinetic left ventricle from the mid ventricle to the apex with mildly impaired global ejection fraction. There was no evidence of myocardial inflammation, infarction, fibrosis or infiltration. A dilated left atrium was also noted. On the basis of these findings a diagnosis of TC was made.
Figure 2.
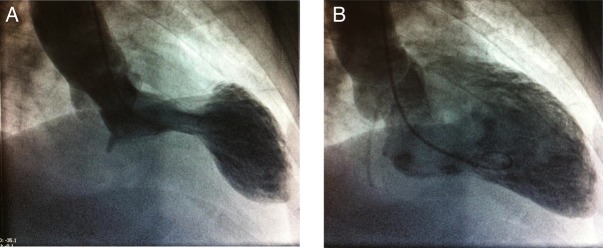
Left ventriculogram during (A) systole and (B) diastole.
Figure 3.
Cardiac MRI.
Differential diagnosis
Incorporated into Investigations section.
Treatment
Bisoprolol 1.25 mg and ramipril 2.5 mg were started for cardiac failure and rhythm disturbance.
Outcome and follow-up
The patient was reviewed fortnightly in cardiology clinic and reported no recurrence of his symptoms. A follow-up appointment and echocardiogram was planned for a few months after discharge. However, the patient unfortunately experienced a pathological fracture in November 2013, which proved to be fatal, and a follow-up echocardiogram was never performed to confirm recovery of the myocardium.
Discussion
There is a wide range of cardiovascular toxicities associated with chemotherapy and some immunotherapies, such as ischaemia, vasospasm, arrhythmia, QT prolongation and acute cardiomyopathy.6 Chemotherapy-induced TC remains a relatively new and rare phenomenon. The few cases reported have occurred in association with 5-FU, with isolated cases secondary to cetuximab, oxaliplatin and capecitabine.1 7
Rituximab is associated with a diverse range of side effects, but unlike its more cardiotoxic monoclonal antibodies such as transtuzumab, its cardiac side effect profile is less well recognised.8 9 Eighty seven per cent of patients experience infusion-related side effects, such as fevers, chills and rigours; these commonly occur within the first few hours, especially during the first infusion. Less severe reactions such as hypotension, angioedema, hypoxia or bronchospasm can be seen in up to 10% of cases.10 These symptoms are associated with cytokine release, particularly IL-6. Most of these cases resolve promptly with supportive management.
Cardiovascular toxicity in the form of cardiac dysrhythmias has been reported in 8% of patients treated with rituximab.8 These include monomorphic ventricular tachycardia, supraventricular tachycardia, trigeminy and irregular pulse, and an isolated case of a fatal infusion secondary to myocardial infarction.11–14
The pathophysiology of TC in our reported case remains a subject of debate. While the aetiology of TC is not fully understood, most investigators believe that the conceptual basis behind these changes is neurohormonal activation causing excessive sympathetic stimulation. This in turn causes microvascular dysfunction at the level of the coronary arteries and transient vasospasm causing transient loss of blood supply to the myocardium. This would be in keeping with observations that they occur most during periods of emotional and physical stress.15
There is growing evidence that the pathophysiology may be more complex and could involve direct myocardial injury. Kanamori et al reported increased ventricular dysfunction after an infusion of rituximab. After infusion, cardiomyocytes were observed to have diffuse amounts of reticulin fibre along with increased serum transforming growth factor-β levels. The authors suggested that the transforming growth factor-β levels could have led to increased reticulin fibre formation causing a decrease in myocardial contractility and conduction.16
An interesting parallel to this is the 2011 literature review on 5-FU-induced TC.1 Grunwald et al highlighted human and autopsy studies showing that myocarditis is a plausible aetiology for cardiomyopathy.17–19 This hypothetical pathophysiology could be supported by the observation that the global/apical hypokinesis observed in TC may be too diffuse to be explained by coronary vasospasms.
However, Lee and Kukreti20 in 2011 claimed that they reported the first case of coronary vasospasm following rituximab infusion. Their patient presented in a similar way to ours, with very similar ECG and angiogram findings, but an echocardiogram was not performed, or at the least not reported. Their basis behind the theorised vasospasm was the quick resolution of the episode with nitroglycerin. While this may lend support to the vasospasm theory, recent evidence may suggest against this. In particular, a negative methylergometrine study in one of the 5-FU case reports have weighed against this more popular theory.21
On the balance of this evidence, we would favour the hypothesis that the pathology occurs at the level of the myocardium rather than the coronary arteries, as far as rituximab-induced TC is involved. This would be in keeping with the case in discussion. We postulate that the reaction occurred during a later infusion as a result of accumulation of reticulin fibres through multiple episodes of rituximab infusion. Unfortunately, an endocardial biopsy was never performed in this patient to confirm this hypothesis.
Learning points.
The first case of its kind to associate rituximab with Takotsubo syndrome.
This case has shed light on the potential of a cytokine-mediated mechanism causing structural changes in the myocardium leading to Takotsubo syndrome.
We propose reconsidering the cardiac side effect profile of this monoclonal antibody as there may be a potential for risk-stratifying recipients of rituximab infusions.
We hope this report has raised awareness among professionals who use rituximab to monitor and respond appropriately to patients who develop cardiac-sounding chest pain while receiving the infusion.
Acknowledgments
The authors would like to thank Dr Simon Davies, Consultant Cardiologist at the Royal Brompton Hospital for providing the left ventriculograms and cardiac MRI reports for this write up.
Footnotes
Contributors: KHN contributed to conception, design, analysis and interpretation of data, drafting of manuscript, and provided final approval; CD contributed to conception and design of work, drafting of manuscript, critical revision of manuscript, and provided final approval; PG contributed to conception, design, analysis and interpretation of data, drafting and critical revision of the manuscript, and provided final approval.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Grunwald MR, Howie L, Diaz LA Jr. Takotsubo syndrome and fluorouracil: case report and review of the literature. J Clin Oncol 2012;30:e11–14. 10.1200/JCO.2011.38.5278 [DOI] [PubMed] [Google Scholar]
- 2.Prasad A. Apical ballooning syndrome: an important differential diagnosis of acute myocardial infarction. Circulation 2007;115:e56–9. 10.1161/CIRCULATIONAHA.106.669341 [DOI] [PubMed] [Google Scholar]
- 3.Tsuchihashi K, Ueshima K, Uchida T et al. Transient left ventricular apical ballooning without coronary artery stenosis: a novel heart syndrome mimicking acute myocardial infarction. J Am Coll Cardiol 2001;38:11–18. 10.1016/S0735-1097(01)01316-X [DOI] [PubMed] [Google Scholar]
- 4.Gianni M, Dentali F, Grandi AM et al. Apical ballooning syndrome or Takotsubo cardiomyopathy: a systematic review. Eur Heart J 2006;27:1523–9. 10.1093/eurheartj/ehl032 [DOI] [PubMed] [Google Scholar]
- 5.Kurisu S, Sato H, Kawagoe T et al. Tako-tsubo-like left ventricular dysfunction with ST-segment elevation: a novel cardiac syndrome mimicking acute myocardial infarction. Am Heart J 2002;143:448–55. 10.1067/mhj.2002.120403 [DOI] [PubMed] [Google Scholar]
- 6.Yeh ET, Tong AT, Lenihan DJ et al. Cardiovascular complications of cancer therapy: diagnosis, pathogenesis, and management. Circulation 2004;109:3122–31. 10.1161/01.CIR.0000133187.74800.B9 [DOI] [PubMed] [Google Scholar]
- 7.Kim L, Karas M, Wong SC. Chemotherapy-induced Takotsubo cardiomyopathy. J Invasive Cardiol 2008;20:E338–40. [PubMed] [Google Scholar]
- 8.Foran JM, Rohaitner AZ, Cunningham D et al. European Phase II study of rituximab (chimeric anti-CD20 monoclonal antibody) for patients with newly diagnosed mantle cell lymphoma and previously treated mantle cell lymphoma, immunocytoma, and small B lymphocytic lymphoma. J Clin Oncol 2000;18:317–24. [DOI] [PubMed] [Google Scholar]
- 9.Killickap S, Yavuz B, Aksoy S et al. Addition of rituximab to chop does not increase the risk of cardiotoxicity in patients with non-Hodgkin's lymphoma. Med Oncol 2008;25:437–42. 10.1007/s12032-008-9062-2 [DOI] [PubMed] [Google Scholar]
- 10.Cercosimo RJ. Monoclonal antibodies in the treatment of cancer, part 2. Am J Health Syst Pharm 2003;60:1631–41. [PubMed] [Google Scholar]
- 11.Arunprasath P, Gobu P, Dubashi B et al. Rituximab induced myocardial infarction: a fatal drug reaction. J Cancer Res Ther 2011;7:346–8. 10.4103/0973-1482.87003 [DOI] [PubMed] [Google Scholar]
- 12.Arai Y, Tadokoro J, Mitani K. Ventricular tachycardia associated with infusion of rituximab in mantle cell lymphoma. Am J Hematol 2005;78:317–18. 10.1002/ajh.20303 [DOI] [PubMed] [Google Scholar]
- 13.Dillman RO. Infusion reactions associated with the therapeutic use of monoclonal antibodies in the treatment of malignancy. Cancer Metastasis Rev 1999;18:465–71. 10.1023/A:1006341717398 [DOI] [PubMed] [Google Scholar]
- 14. Rituxan official FDA information, side effects and uses. March 2007 (cited 2 Mar 2010). http:www.drugs.com/pro/rituxan.html.
- 15.Wittstein IS, Thiemann DR, Lima JA et al. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med 2005;352:539–48. 10.1056/NEJMoa043046 [DOI] [PubMed] [Google Scholar]
- 16.Kanamori H, Tsutsumi Y, Mori A et al. Delayed reduction in left ventricular function following treatment of non-Hodgkin's lymphoma with chemotherapy and rituximab, unrelated to acute infusion reaction. Cardiology 2006;105:184–7. 10.1159/000091416 [DOI] [PubMed] [Google Scholar]
- 17.Martin M, Diaz-Rubio E, Furio V et al. Lethal cardiac toxicity after cisplatin and 5-fluorouracil chemotherapy: report of a case with necropsy study. Am J Clin Oncol 1989;12:229–34. 10.1097/00000421-198906000-00010 [DOI] [PubMed] [Google Scholar]
- 18.Kuropkat C, Griem K, Clark J et al. Severe cardiotoxicity during 5-fluorouracil chemotherapy: a case and literature report. Am J Clin Oncol 1999;22:466–70. 10.1097/00000421-199910000-00009 [DOI] [PubMed] [Google Scholar]
- 19.Tsibiribi P, Bui-Xuan C, Bui-Xuan B et al. Cardiac lesions induced by 5-fluorouracil in the rabbit. Hum Exp Toxicol 2006;25:305–9. 10.1191/0960327106ht628oa [DOI] [PubMed] [Google Scholar]
- 20.Lee L, Kukreti V. Rituximab-induced coronary vasopasm. Case Rep Hematol 2012;2012:984986 http://www.hindawi.com/crim/hematology/2012/984986/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Basselin C, Fontanges T, Descotes J et al. 5-Fluorouracil-induced Takotsubo-like syndrome. Pharmacotherapy 2011;31:226 10.1592/phco.31.2.226 [DOI] [PubMed] [Google Scholar]