Figure 2.
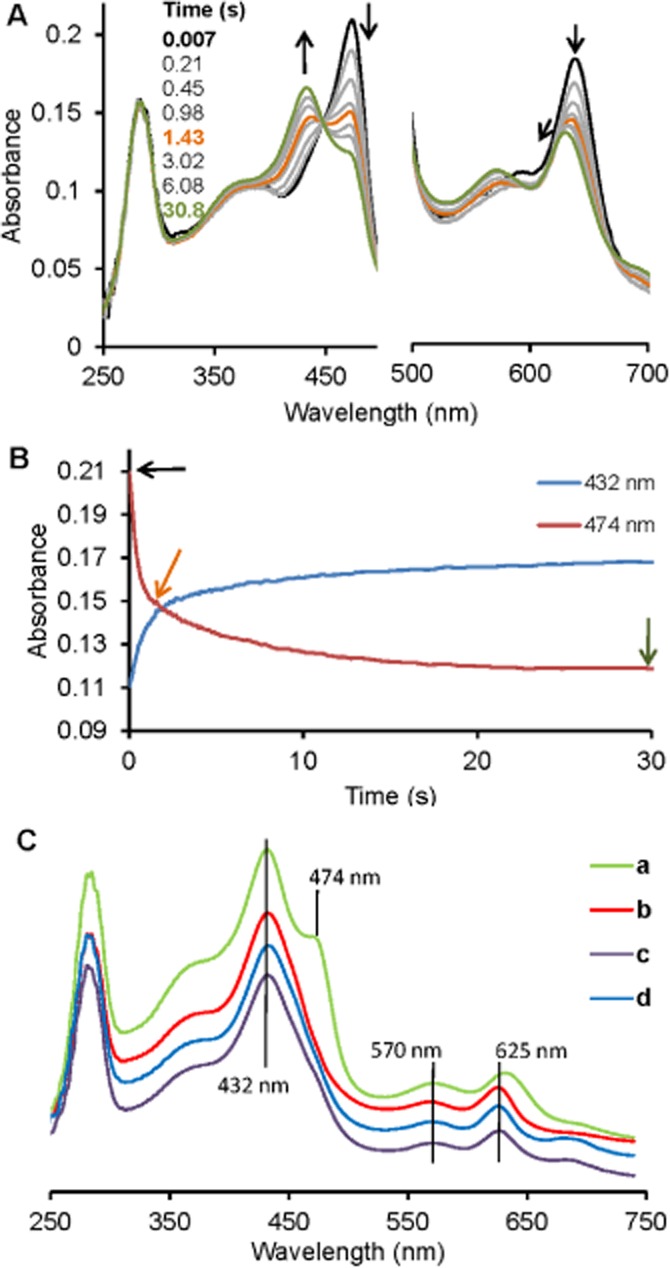
Kinetics of sulfide binding to ferrous MPO monitored by stopped-flow spectroscopy. (A) Spectral changes upon the reaction of 2 µM ferrous MPO with 125 µM sulfide at 25°C in 50 mM phosphate buffer at pH = 7.4 in a conventional stopped-flow experiment. The first spectrum was recorded 7 ms after mixing, with subsequent spectra taken at the indicated time points. The bold black spectrum corresponds to ferrous MPO and arrows indicate the direction of the spectral transitions. The black spectrum was taken at 7 ms, the orange at 1.43 s and the green at 30.8 s. (B) Time traces at 474 and 432 nm, correspond to spectral transitions at (A) with arrows indicating time points where the highlighted spectra in (A) were recorded: (C) Comparison of the spectra of reaction products obtained from the reactions of sulfide with ferrous MPO (a), ferric MPO (b), compound I (c) or compound II (d) at 30.8 s (a), 259 s (b), 1.9 s (c) and 1 s (d) time points.
