Figure 8.
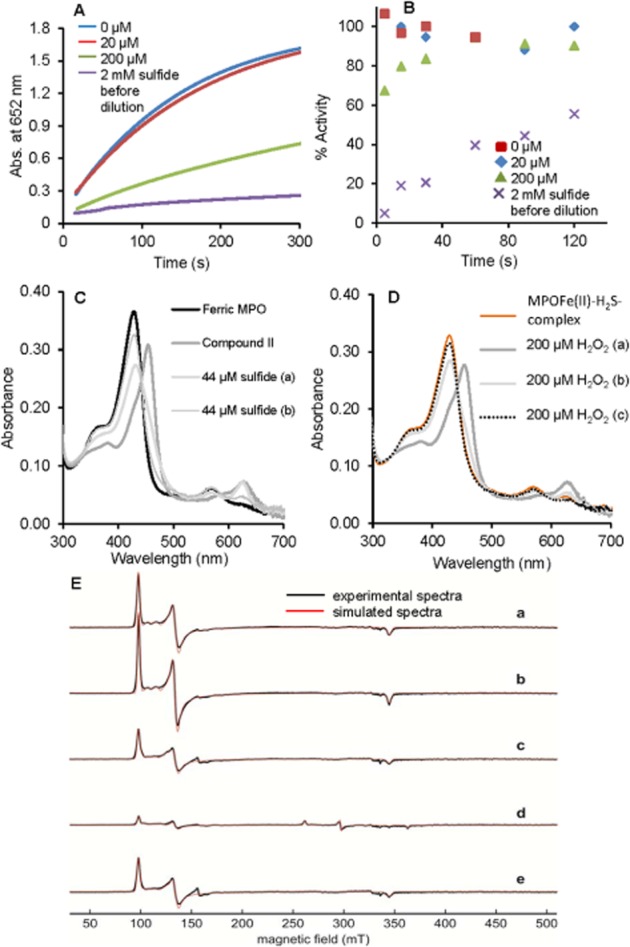
Reversible sulfide inhibition of MPO peroxidase activity. (A) The mixtures of 2 µM MPO with 0 µM (blue), 20 µM (red line), 200 µM (green line) or 2000 µM (purple line) sulfide were diluted 333-fold to a final enzyme concentration of 6 nM (at 25°C and pH 7.4 in 100 mM phosphate buffer). The peroxidase activity of the differently treated MPO solutions were measured as described ∼2 min after dilution. (B) Time-dependent change of the peroxidase activity in the diluted (333-fold as in A) reaction mixtures for 0 µM, 20 µM, 200 µM or 2000 µM sulfide pretreatment. (C) Reaction of MPO compound II with sulfide. Compound II was formed by reaction of 4 µM ferric MPO in 100 mM phosphate buffer pH 7.4 with 70-fold excess of H2O2. Excess of H2O2 was removed after 35 s by adding 10 µg mL−1 bovine liver catalase. Compound II was mixed with 44 µM sulfide and consecutive spectra were obtained at 30 s (trace a) and 10 min (trace b). (D) To the resulting MPO–sulfide complexes that were generated in the reaction of compound II with sulfide (in the experiment shown in C) 200 µM H2O2 was added and subsequent spectra were recorded after 30 s (trace a), 2 min (trace b) and 10 min (trace c). (E) Experimental cw EPR spectra and simulations of 100 µM MPO (a), or 100 µM MPO with (b) 100 µM H2O2, (c) 10 mM H2O2, (d) 100 µM H2O2 and 440 µM sulfide, or (e) 10 mM H2O2 and 550 µM sulfide, at pH 7.4. The spectra were recorded at 10 K.
