Figure 2.
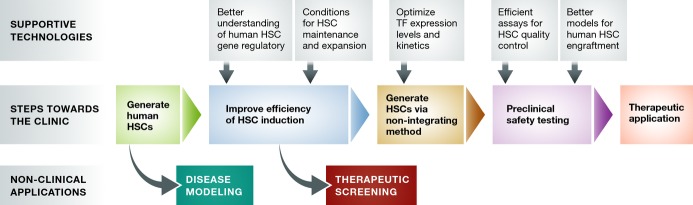
Road map to clinical translation of human induced HSCs
Upon establishment of a method for generating human HSCs from patient-derived cells, a number of critical steps must be taken en route to clinical translation. Although the initial, suboptimal method may be sufficient for initiating patient-specific disease modeling research, substantial improvements in the efficiency of HSC generation must be made to obtain sufficient numbers of HSCs toward therapeutic screening and for use in reconstituting adult patients. Prior to preclinical testing, a non-integrating approach to generating HSCs must be established in order to eliminate the risks associated with insertional mutagenesis and accidental re-induction of reprogramming TFs, many of which are potently oncogenic. During the preclinical phase, quality control and safety testing should be performed at molecular and functional levels. Functional testing should involve the best methods for modeling human HSC engraftment such as humanized immunodeficient animal recipients.
