Abstract
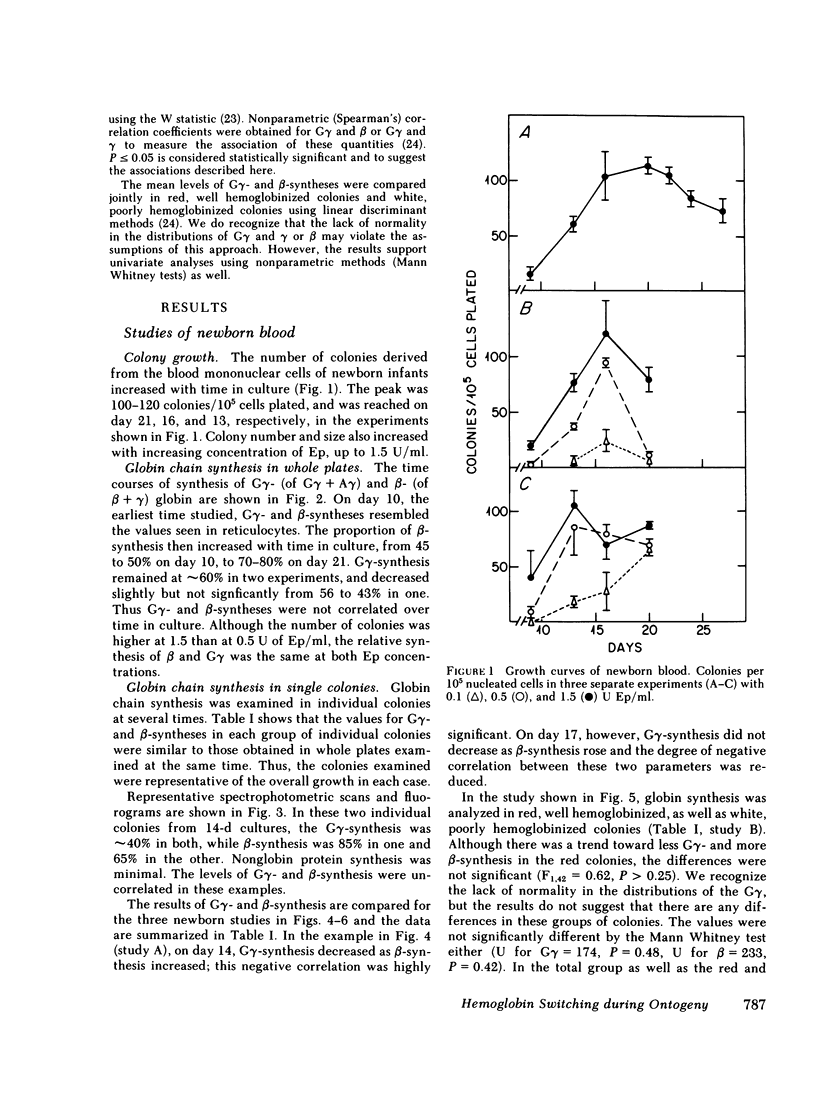
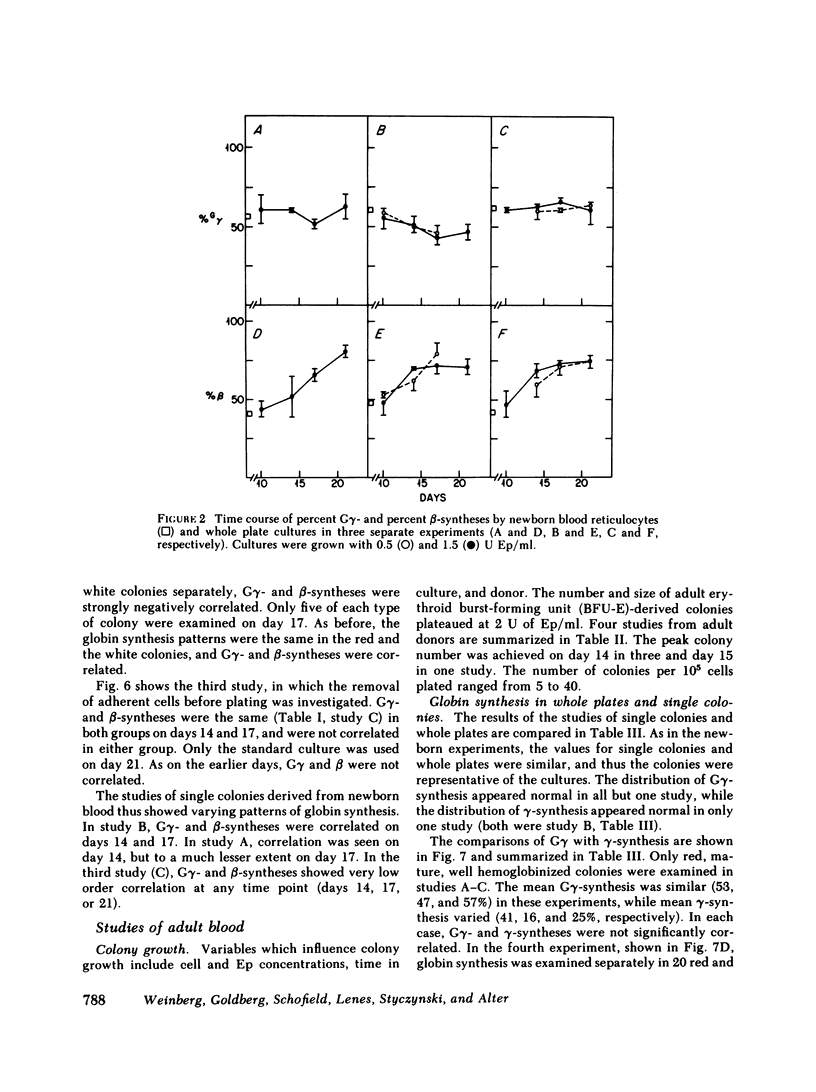
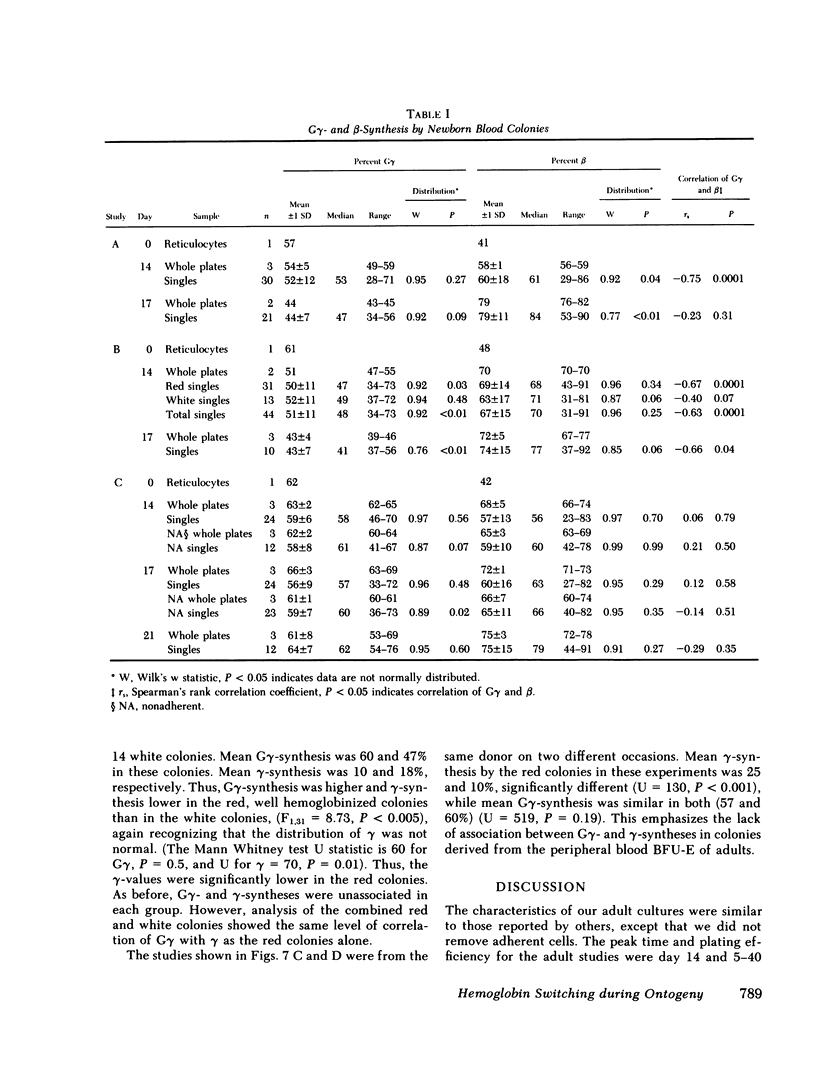
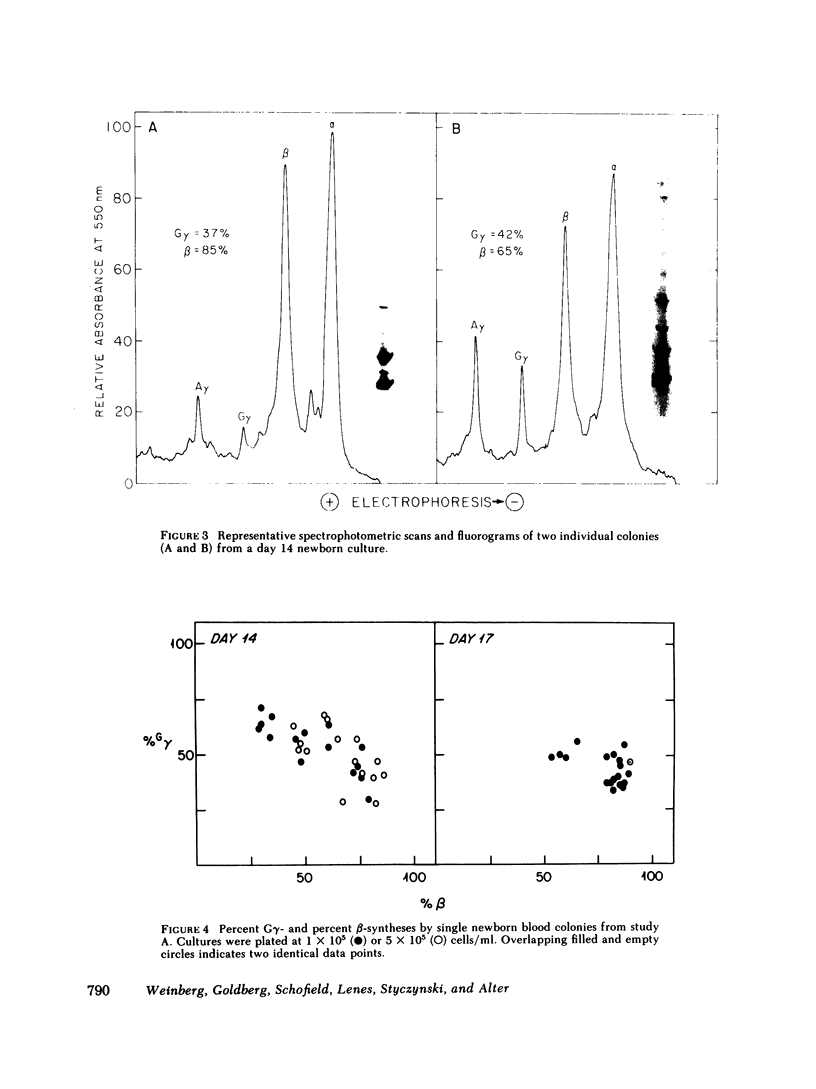
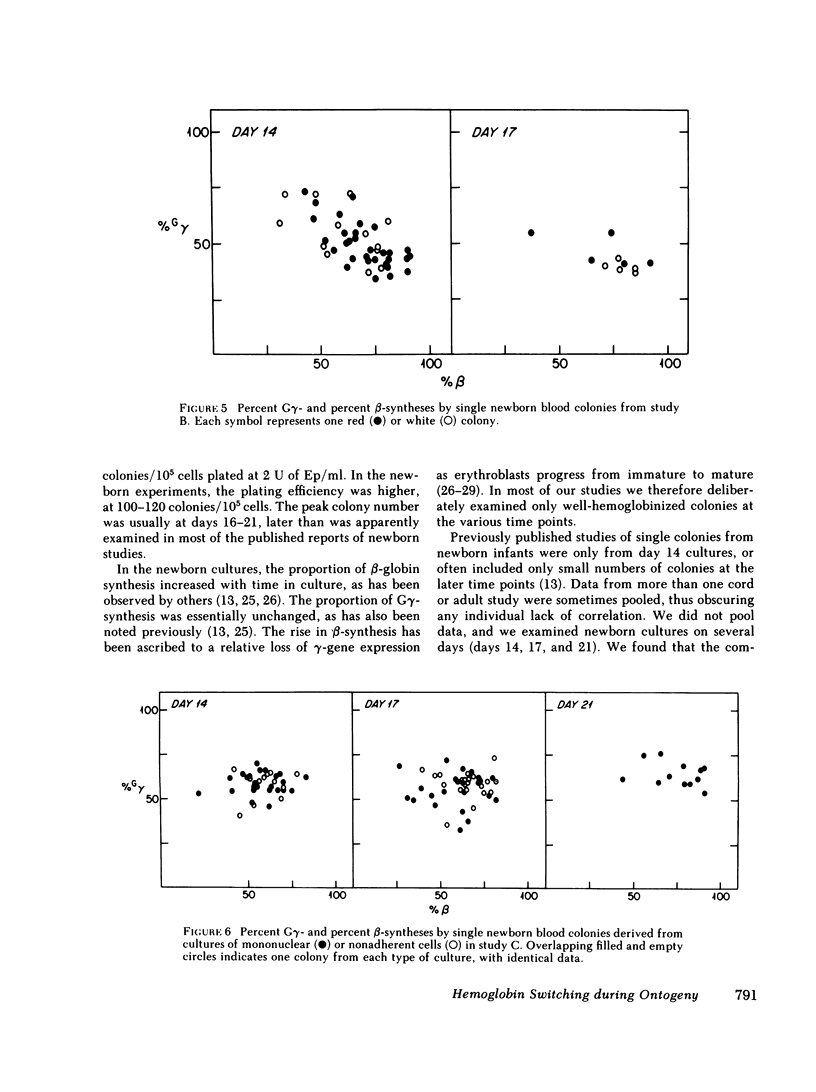
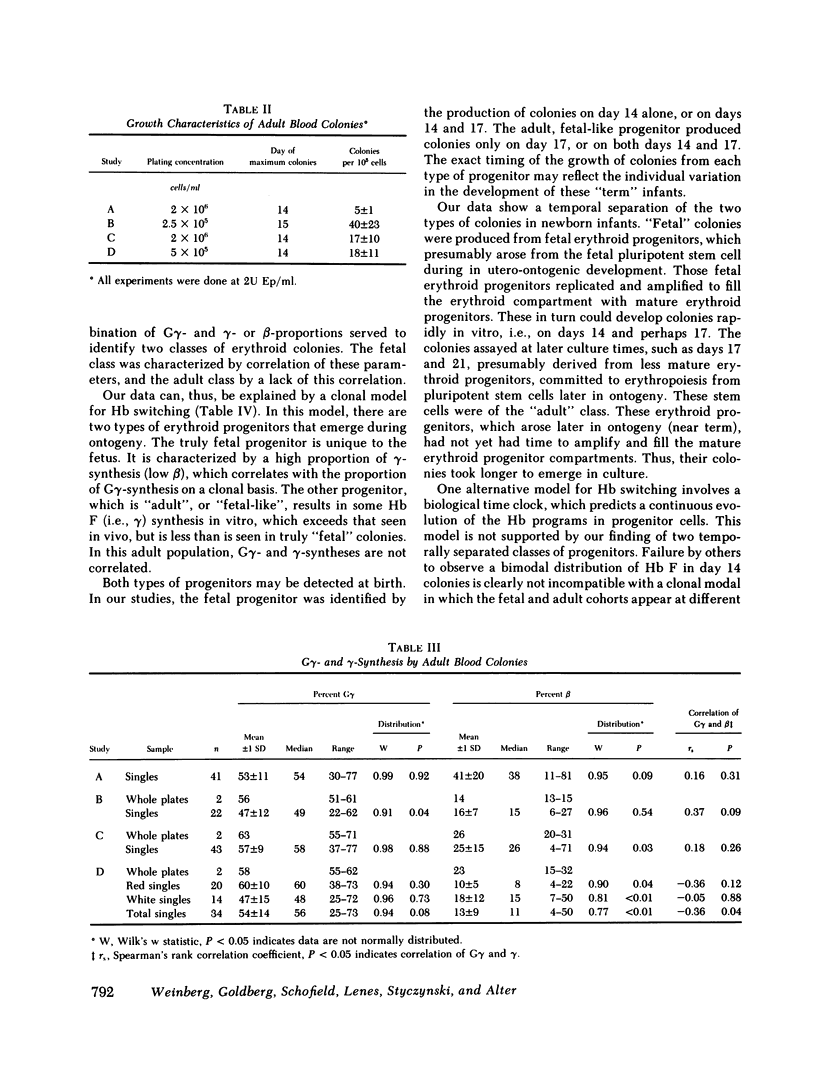
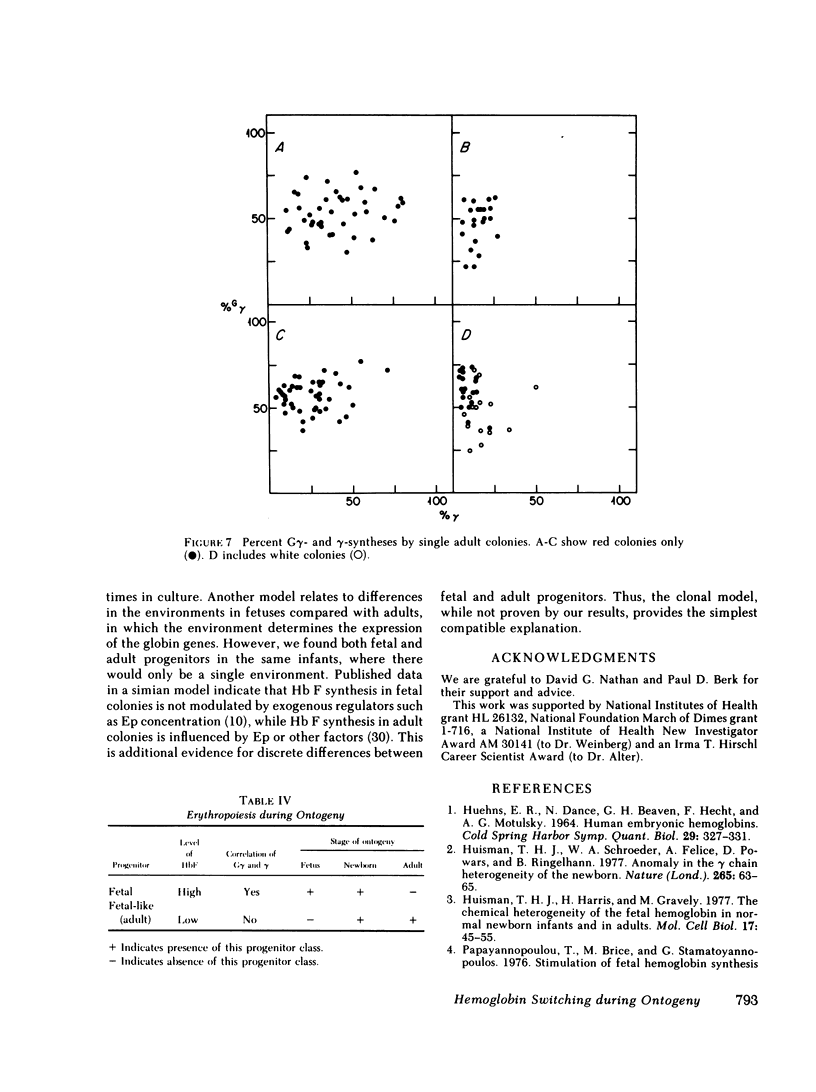
To examine the switch from fetal to adult hemoglobin at the cellular level, erythroid progenitor cells from newborn infants and adults were cultured in methyl cellulose with erythropoietin. Individual erythroid colonies were labeled with [3H]leucine at various times, and globin synthesis patterns examined by gel electrophoresis and fluorography. The percent gamma- or beta-globin synthesis was determined from the total of gamma + beta, and the percent G gamma from the total of G gamma + A gamma. The nonparametric correlation coefficients of percent G gamma with percent gamma or beta were obtained. Each group of colonies at each time point was examined separately. In colonies from adult blood, the proportion of G gamma-synthesis did not correlate with the proportion of gamma-synthesis. Colonies from newborn blood fell into two groups. Those that developed from relatively mature progenitor cells, and were seen on day 14, showed a strong negative correlation of G gamma with beta-globin synthesis. However, those newborn colonies that developed from immature progenitors, and were seen later in culture (days 17 and 21), showed no correlation of G gamma with beta-synthesis. These findings are compatible with a clonal model for hemoglobin switching. Fetal progenitors, in which G gamma- and beta-syntheses are negatively correlated, are gradually replaced during ontogeny by adult progenitors. The adult progenitors produce more beta (less gamma), and the proportions of G gamma- and gamma- or beta-synthesis are not correlated.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alter B. P., Goff S. C., Efremov G. D., Gravely M. E., Huisman T. H. Globin chain electrophoresis: a new approach to the determination of the G gamma/A gamma ratio in fetal haemoglobin and to studies of globin synthesis. Br J Haematol. 1980 Apr;44(4):527–534. doi: 10.1111/j.1365-2141.1980.tb08706.x. [DOI] [PubMed] [Google Scholar]
- Alter B. P., Jackson B. T., Lipton J. M., Piasecki G. J., Jackson P. L., Kudisch M., Nathan D. G. Control of the simian fetal hemoglobin switch at the progenitor cell level. J Clin Invest. 1981 Feb;67(2):458–466. doi: 10.1172/JCI110054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alter B. P., Modell C. B., Fairweather D., Hobbins J. C., Mahoney M. J., Frigoletto F. D., Sherman A. S., Nathan D. G. Prenatal diagnosis of hemoglobinopathies. A review of 15 cases. N Engl J Med. 1976 Dec 23;295(26):1437–1443. doi: 10.1056/NEJM197612232952601. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Comi P., Giglioni B., Ottolenghi S., Gianni A. M., Polli E., Barba P., Covelli A., Migliaccio G., Condorelli M., Peschle C. Globin chain synthesis in single erythroid bursts from cord blood: studies on gamma leads to beta and G gamma leads to A gamma switches. Proc Natl Acad Sci U S A. 1980 Jan;77(1):362–365. doi: 10.1073/pnas.77.1.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darbre P. D., Lauckner S. M., Adamson J. W., Wood W. G., Weatherall D. J. Haemoglobin synthesis in human erythroid bursts during ontogeny: reproducibility and sensitivity to culture conditions. Br J Haematol. 1981 Jun;48(2):237–250. [PubMed] [Google Scholar]
- Dean A., Schechter A. N., Papayannopoulou T., Stamatoyannopoulos G. Heterogeneity of erythroid precursor cells. Hemoglobin quantitation in single clones by radioimmunoassay. J Biol Chem. 1981 Mar 10;256(5):2447–2453. [PubMed] [Google Scholar]
- Dover G. J., Boyer S. H. Quantitation of hemoglobins within individual red cells: asynchronous biosynthesis of fetal and adult hemoglobin during erythroid maturation in normal subjects. Blood. 1980 Dec;56(6):1082–1091. [PubMed] [Google Scholar]
- HUEHNS E. R., DANCE N., BEAVEN G. H., HECHT F., MOTULSKY A. G. HUMAN EMBRYONIC HEMOGLOBINS. Cold Spring Harb Symp Quant Biol. 1964;29:327–331. doi: 10.1101/sqb.1964.029.01.035. [DOI] [PubMed] [Google Scholar]
- Huisman T. H., Harris H., Gravely M. The chemical heterogeneity of the fetal hemoglobin in normal newborn infants and in adults. Mol Cell Biochem. 1977 Aug 19;17(1):45–55. doi: 10.1007/BF01732554. [DOI] [PubMed] [Google Scholar]
- Huisman T. H., Schroeder W. A., Felice A., Powars D., Ringelhann B. Anomaly in the gamma chain heterogeneity of the newborn. Nature. 1977 Jan 6;265(5589):63–65. doi: 10.1038/265063a0. [DOI] [PubMed] [Google Scholar]
- Iscove N. N., Sieber F., Winterhalter K. H. Erythroid colony formation in cultures of mouse and human bone marrow: analysis of the requirement for erythropoietin by gel filtration and affinity chromatography on agarose-concanavalin A. J Cell Physiol. 1974 Apr;83(2):309–320. doi: 10.1002/jcp.1040830218. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lipton J. M., Nathan D. G. Cell-cell interactions in in vitro erythropoiesis. Blood Cells. 1980;6(4):645–663. [PubMed] [Google Scholar]
- Macklis R. M., Javid J., Lipton J. M., Kudisch M., Pettis P. K., Nathan D. G. Synthesis of hemoglobin F in adult simian erythroid progenitor-derived colonies. J Clin Invest. 1982 Oct;70(4):752–761. doi: 10.1172/JCI110671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papayannopoulou T. H., Brice M., Stamatoyannopoulos G. Stimulation of fetal hemoglobin synthesis in bone marrow cultures from adult individuals. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2033–2037. doi: 10.1073/pnas.73.6.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papayannopoulou T., Brice M., Stamatoyannopoulos G. Hemoglobin F synthesis in vitro: evidence for control at the level of primitive erythroid stem cells. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2923–2927. doi: 10.1073/pnas.74.7.2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papayannopoulou T., Kalmantis T., Stamatoyannopoulos G. Cellular regulation of hemoglobin switching: evidence for inverse relationship between fetal hemoglobin synthesis and degree of maturity of human erythroid cells. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6420–6424. doi: 10.1073/pnas.76.12.6420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papayannopoulou T., Kurachi S., Brice M., Nakamoto B., Stamatoyannopoulos G. Asynchronous synthesis of HbF and HbA during erythroblast maturation. II. Studies of G gamma, A gamma, and beta chain synthesis in individual erythroid clones from neonatal and adult BFU-E cultures. Blood. 1981 Mar;57(3):531–536. [PubMed] [Google Scholar]
- Papayannopoulou T., Nakamoto B., Kurachi S., Stamatoyannopoulos G. Globin synthesis in erythroid bursts that mature sequentially in culture. I. Studies in cultures of adult peripheral blood BFU-Es. Blood. 1981 Nov;58(5):969–974. [PubMed] [Google Scholar]
- Rinehart J. J., Zanjani E. D., Nomdedeu B., Gormus B. J., Kaplan M. E. Cell-cell interaction in erythropoiesis. Role of human monocytes. J Clin Invest. 1978 Nov;62(5):979–986. doi: 10.1172/JCI109227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatoyannopoulos G., Kurnit D. M., Papayannopoulou T. Stochastic expression of fetal hemoglobin in adult erythroid cells. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7005–7009. doi: 10.1073/pnas.78.11.7005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasawa T., Ogawa M. Hemoglobin biosynthesis in individual bursts from human adult peripheral and umbilical cord blood: analysis of the relative rates of synthesis of G gamma and A gamma globin chains. J Cell Physiol. 1980 Dec;105(3):483–488. doi: 10.1002/jcp.1041050312. [DOI] [PubMed] [Google Scholar]
- Terasawa T., Ogawa M., Porter P. N., Karam J. D. G gamma and A gamma globin-chain biosynthesis by adult and umbilical cord blood erythropoietic bursts and reticulocytes. Blood. 1980 Jul;56(1):93–97. [PubMed] [Google Scholar]
- Worton R. G., McCulloch E. A., Till J. E. Physical separation of hemopoietic stem cells from cells forming colonies in culture. J Cell Physiol. 1969 Oct;74(2):171–182. doi: 10.1002/jcp.1040740209. [DOI] [PubMed] [Google Scholar]



