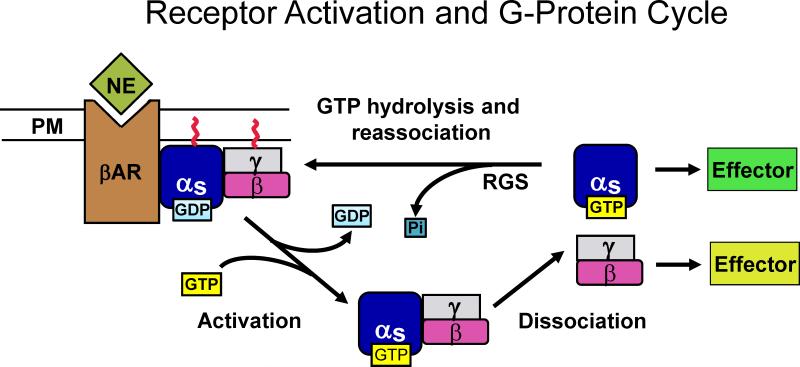
Figure 1.
β-Adrenergic receptor activation and the G-protein cycle. In the resting state, the heterotrimeric G-protein, Gs, is bound to the β-adrenergic receptor (βAR), and anchored to the plasma membrane (PM) via covalently attached lipid molecules. Norepinephrine (NE) released from sympathetic nerve terminals in the salivary glands binds to the βAR, resulting in activation of Gs. Cytoplasmic GTP is exchanged for GDP on the Gα subunit, causing release of Gs from the receptor and dissociation of the Gα and Gβγ subunits. In salivary gland acinar cells Gα then activates the effector protein, adenylate cyclase, which forms cAMP from ATP. Gβγ may activate other effector proteins. Hydrolysis of GTP, accelerated by regulator of G-protein signaling (RGS) proteins, results in reassociation of Gα and Gβγ, and subsequent reassociation of Gs with the βAR. (For color reproduction on the Web and in print.)