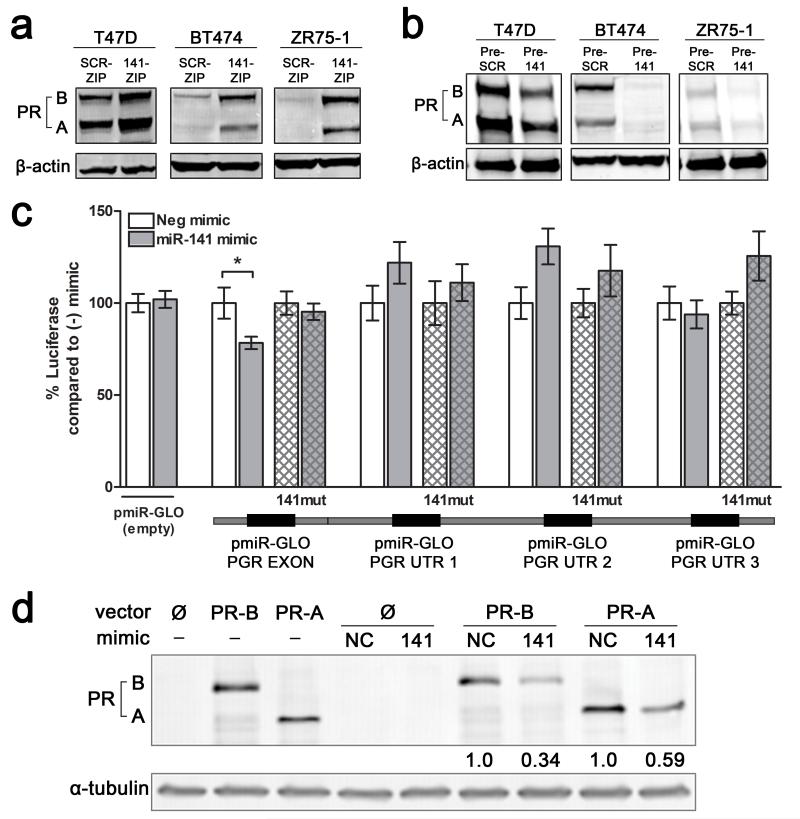
Figure 4.
miR-141 regulates PR expression levels in luminal breast cancer cell lines and directly targets the PR transcript. (a) Stable inhibition of miR-141 increases PR expression. PR expression was measured by Western blot in untreated T47D, BT474, or ZR75-1 cells with stable inhibition of miR-141 (141-ZIP) or scrambled control (SCR-ZIP). PR-A and PR-B isoforms are indicated. β-actin was used as loading control. (b) Stable overexpression of miR-141 decreases endogenous PR expression. PR expression measured by Western blot in untreated T47D, BT474, or ZR75-1 cells with stable overexpression of miR-141 (Pre-141) or control (Pre-SCR). β-actin was used as loading control. (c) miR-141 directly targets PR through a binding site in the last exon. Predicted miR-141 binding sites in the PR 3′UTR and last exon are outlined below the graph. Regions of the 3′UTR as indicated were cloned singly downstream of luciferase in the pMIR-GLO vector and each site was mutagenized to abolish miR-141 binding. Each luciferase construct or its mutagenized counterpart was transfected into T47D cells with either 50 nM negative control (−) or miR-141 (141) mimic and luciferase activity measured after 48 h. Data represents relative luciferase activity normalized to constitutively active Renilla contained on the same vector. Experiments were repeated twice. Bars are mean +/− SEM; * P<0.05. (d) Plasmids encoding PRB (hPR1) and PRA (hPR2) were transiently transfected into HEK293 cells alone or with negative control (NC) or miR-141 mimics. PR protein levels were measured by Western blot. Fold change of PR compared to NC mimic is indicated; quantification is normalized to α-tubulin.