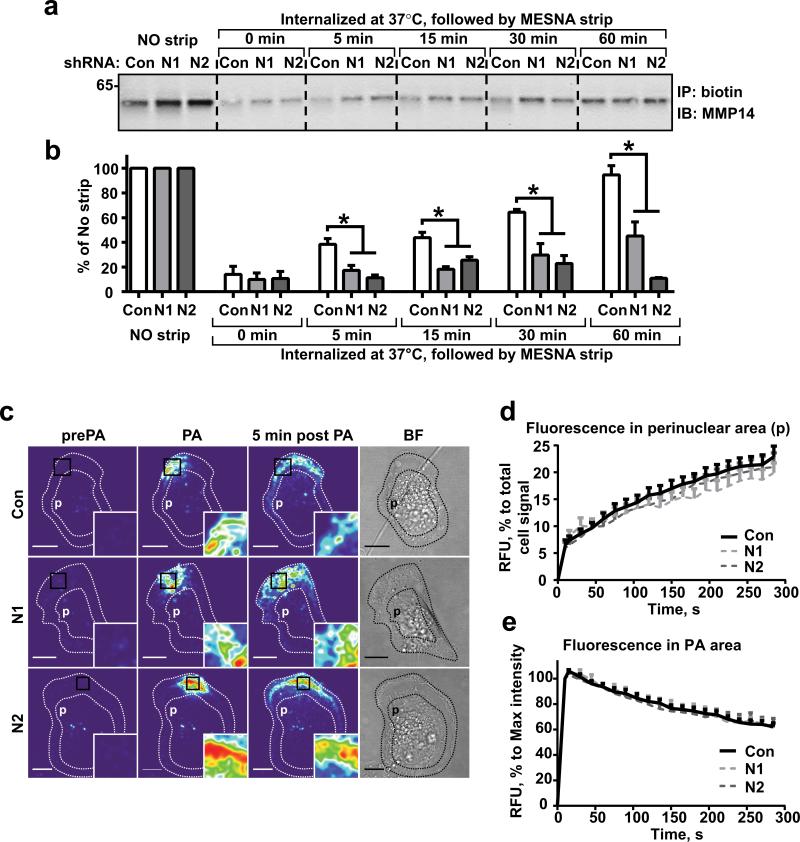
Figure 2. NEDD9 depletion does not affect internalization of MMP14.
(a) Western blot (WB) analysis of immunoprecipitated (IP) biotinylated MMP14 from shCon-, shNEDD9 (N1, N2)-MDA-MB-231cells: no strip-0°C (lines 1-3), the rest incubated at 37°C for indicated times, striped with MESNA, lysed, IPed with streptavidin agarose and probed with anti-MMP14 antibody. (b) Quantification of WBs as in (a), three independent experiments, graphs are mean percent (%) of relative intensity units (RIU) to no strip conditions (100%) ±S.E.M; one-way ANOVA with Dunnett's post-hoc analysis *p= 0.0054, 0.0049, 0.0098, 0.0028 for 5, 15, 30 and 60 min respectively. (c) Representative confocal images of shCon-, shNEDD9 (N1, N2)-MDA-MB-231cells expressing PA-mCherry-MMP14 before photo-activation (pre-PA), after- (PA), and 5 min after PA in a defined area. Scale bar, 10μm; inserts are the enlarged areas of PA; BF-bright field image. (d) Quantification of relative fluorescence intensity units (RFU) of mCherry-MMP14 in cells as in (c) in perinuclear area (p); graph is mean RFU % of total RFU/cell ±S.E.M; 10 cells/per treatment (Con, N1, N2); F test performed for fitted lines, p is non-significant (ns) for shCon/shN1 or /N2. (e) Quantification of relative fluorescence intensity (RFU) of mCherry-MMP14 in cells as in (c) in PA area (black rectangle); graph is mean RFU (%) of max RFU/cell (100%) ±S.E.M; 10 cells/per treatment (Con, N1, N2), F test performed for fitted lines, p is (ns).