Abstract
Background
Ischemia-reperfusion injury (IRI) to the liver continues to be a source of significant morbidity, especially in patients with hepatic steatosis. This is a growing problem given the increase in nonalcoholic fatty liver disease. BH3-only members of the BCL-2 protein family are known mediators of cellular apoptosis, although their role in hepatic IRI is still emerging. The goal of this study was to investigate the effect of Bim and Bid on warm hepatic IRI in the setting of steatosis.
Study Design
Lean and obese Bim/Bid wild type (WT) and double knock out (DKO) mice underwent 60 minutes of warm hepatic ischemia using a 70% segmental occlusion technique. Obesity and hepatic steatosis were induced using a high fat diet. Hepatocellular injury patterns were compared among lean and steatotic mice following reperfusion. Differences were analyzed using a student’s t-test and reported as mean ± SEM.
Results
DKO mice were protected from IRI relative to WT. A high fat diet created equal degrees of steatosis in both WT and DKO mice. The IRI was increased in steatotic WT livers; however, DKO mice remained protected relative to WT despite hepatic steatosis.
Conclusions
The BH3-only proteins are important mediators of hepatic IRI in both lean and steatotic livers. These mechanisms have been underappreciated in steatotic liver injury and may be leveraged as targets for intervention in clinical scenarios such as transplant and hypovolemic shock.
Keywords: Ischemia-reperfusion, Steatosis, Apoptosis, BCL-2 proteins
1.0 INTRODUCTION
Hepatic IRI is a ubiquitous phenomenon that occurs in a variety of clinical settings including liver resection, transplantation and hypovolemic shock. It is well established that certain factors impact the susceptibility of the liver to IRI. Hepatic steatosis is the most prevalent chronic liver disease in the world and its recognition as a major clinical risk factor for IRI has become increasingly important given the epidemic rise of obesity and diabetes. Accentuation of IRI in the setting of steatosis is well described. It has been attributed to mechanisms such as microcirculatory failure [1]. Accumulation of triacylglycerol within hepatocytes increases their volume which distorts sinusoidal architecture and narrows luminal diameter up to 50% that of lean livers [1, 2]. Steatotic livers are also susceptible to increased endoplasmic reticulum stress [3], which leads to multiple pathways of cell death including autophagy [4]. The end result is that multiple alterations in the steatotic liver render it especially susceptible to IRI.
Inflammatory mediators of IRI produce an apoptotic pattern of hepatocellular injury. A ligand for hepatocellular membrane death receptors, circulating TNF-α stimulates activation of intermediaries, which carry the death signal to executioner caspase proteins [5]. Along with the intrinsic pathway of caspase activation, executioner proteins carry out apoptosis through deoxyribose nucleic acid (DNA) destruction, which characterizes the nature of cell death from IRI [6, 7].
The BCL-2 family of proteins regulates apoptosis within cells. Comprised of both pro-apoptotic and anti-apoptotic members, it is the balance of these proteins that ultimately determine the fate of cells under duress. Members are classified according to the number of BCL-2 Homology (BH) domains they share. Bim and Bid are examples of BH3-only proteins, which share only the third BH domain. They are necessary upstream regulators that help to execute apoptosis [5, 8, 9]. Many reports interested in the attenuation of apoptosis have focused on the role of BH3-only proteins. Reduced lymphocyte apoptosis and improved survival from sepsis in both Bim and Bid null mice has led to their therapeutic targeting [11]. In a preclinical model of sepsis, small interfering ribonucleic acid (siRNA) silencing of Bim significantly reduced lymphocyte apoptosis and conferred a survival advantage in the treated cohort [12]. However, the role of BH3-only proteins in hepatic IRI remains poorly understood, especially in the steatotic liver. In the present study, we hypothesized that absence of Bim and Bid would have a protective effect on the liver during IRI. Further, we investigated whether this hypothesized protection would be impacted by hepatic steatosis.
2.0 MATERIAL AND METHODS
2.1 Animals
The study was conducted with approval from the Animal Studies Committee at Washington University in Saint Louis, which promotes the welfare of animals utilized for experimentation in accordance with the Guide for the Care and Use of Laboratory Animals [13]. Generation of Bim (−/−), Bid (−/−) (Double Knockout, DKO) mice were achieved through standard breeding protocols. Bim (−/−) and Bid (−/−) mating pairs were the generous gift of Dr. Richard Hotchkiss, MD [11]. All mice were on a C57BL6 background. Genotyping was carried out by polymerase chain reaction using tail snips. C57BL6 Bim (+/+), Bid (+/+) WT mice served as controls and were obtained from Jackson Laboratories. These animals were maintained in the Washington University in St. Louis animal facility.
2.2 Model of In Vivo Hepatic IRI
Age and sex matched Bim/Bid WT and DKO mice were given 3.0% isoflurane. Following induction of general anesthesia, maintenance isoflurane was delivered via nose cone at 1.5%. Under sterile technique with 2.5x loupe magnification, the abdomen was entered via a midline laparotomy. With gentle retraction of the liver, the caudate ligament was divided and the portal vessels feeding the left lateral and median lobes identified. An atraumatic vascular clamp was then applied to produce 70% hepatic ischemia for a period of 60 minutes. Segmental ischemia was chosen to reduce mesenteric congestion and the complications that result from total hepatic IR [14, 15]. Animals experienced 6 hours of reperfusion. Euthanasia took place by cardiac puncture. Serum and liver tissue were collected for transaminase content, histology, and immunohistochemistry.
2.3 Model of diet-induced hepatic steatosis
Following previously established protocols, WT and DKO mice aged 9 – 11 weeks were fed a high fat diet (HFD) (60% of kilocalories from fat; Research Diets D12492) ad libitum for a period of 5 weeks [16]. WT and DKO mice fed standard chow served as controls for IRI, weight, as well as hepatic triglyceride content.
2.4 Serum analysis
Whole blood collected in 1 cc heparin coated syringes via cardiac puncture was centrifuged for 10 minutes at 13,000 rpm. Serum for immediate analysis was stored overnight at −4° C, whereas aliquots for later analysis were kept frozen at − 80° C. Serum levels of aspartate aminotransferase (AST) served as a surrogate marker for hepatocellular injury, which was assessed using standard chemistry analyzers.
2.5 Histopathology and Immunohistochemistry
Following cardiac puncture, liver tissue was procured and fixed in 10% formalin. Tissue was then processed and stained with hematoxylin-eosin to assess the severity of inflammation following warm hepatic IRI. Staining for surrogate markers of apoptosis, TUNEL and activated Caspase-3, was performed to assess the nature of hepatocellular injury. Imaging software (NIS-Elements, Nikon) aided in differentiation through utilization of a consistent positive threshold.
2.6 Hepatic triglyceride content
Following cardiac puncture, liver tissue was procured and frozen in liquid nitrogen (−196 °C). Tissue was then processed and stained with Oil Red O to assess for steatosis in mice following HFD protocol. In addition, lipids from homogenized liver tissue were extracted and a commercially available enzymatic assay kit (L-Type TG H, Wako Diagnostics) was utilized to quantify TG content. Absorbance was plotted against the standard curve to give TG concentration in µg/mg of protein.
2.7 Statistical analysis
Statistical analyses were conducted using GraphPad software, San Diego CA. Study cohorts were screened for statistical outliers using Grubbs’ test. Significant outliers (p < 0.05) were excluded from comparison, which was performed using a Student’s t-test. Results are presented as mean ± SEM. A p value of ≤ 0.05 was considered significant.
3.0 RESULTS
3.1 BH3-only proteins are deleterious in IRI
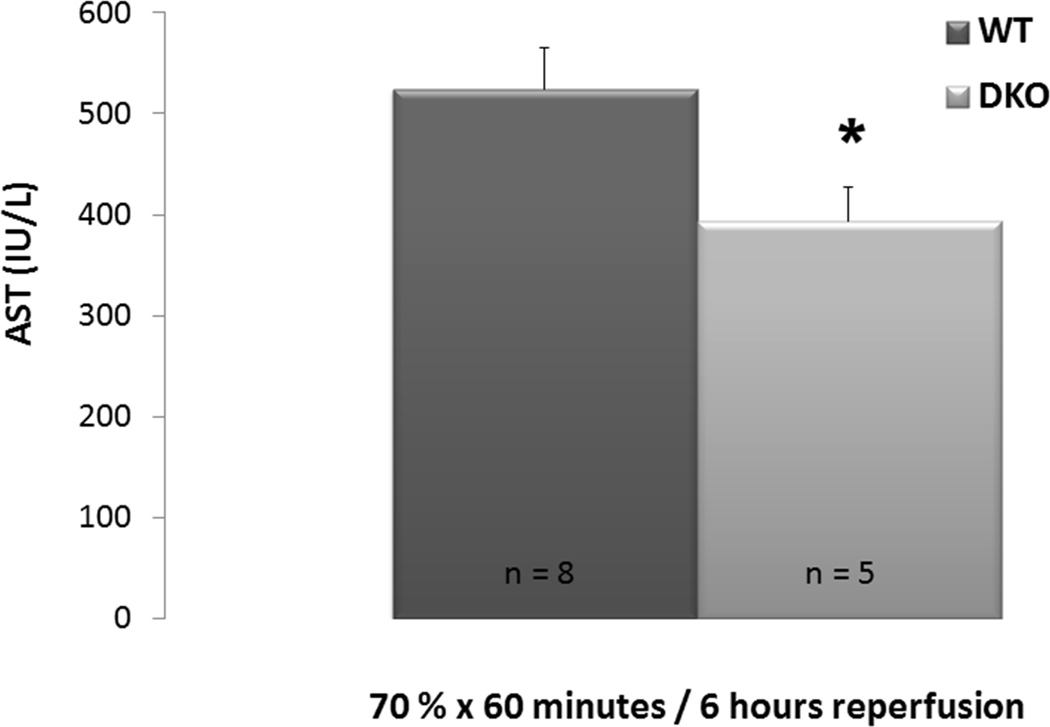
Lean WT and DKO mice fed standard chow underwent 70% hepatic inflow occlusion for 60 minutes by microvascular clamping. Following 6 hours of reperfusion, animals were euthanized for collection of serum. DKO mice were protected from IRI relative to the WT controls (AST: 524.9 IU/L ±40.0 vs. 393.2 IU/L ±34.2, p = 0.04, figure 1).
Figure 1. Absence of BH3-only proteins mitigates IRI in lean mice.
WT and DKO mice underwent 60 minutes of 70% hepatic ischemia in vivo followed by 6 hours of reperfusion. Hepatic transaminase release was significantly reduced in WT mice compared to DKO.
3.2 Diet-induced hepatic steatosis is not affected by the absence of BH3-only proteins
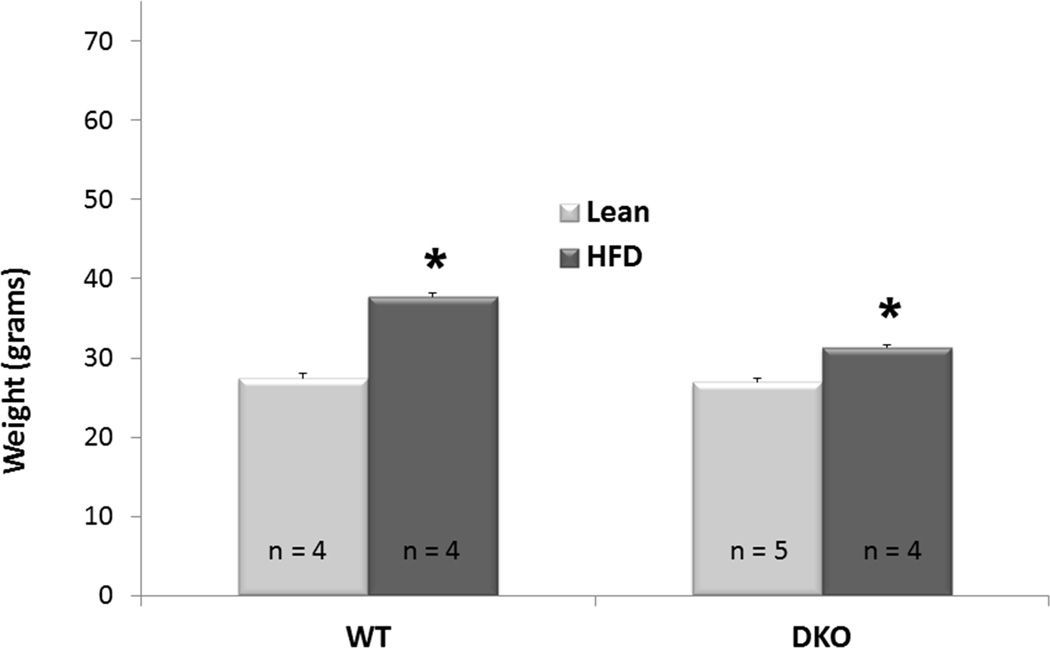
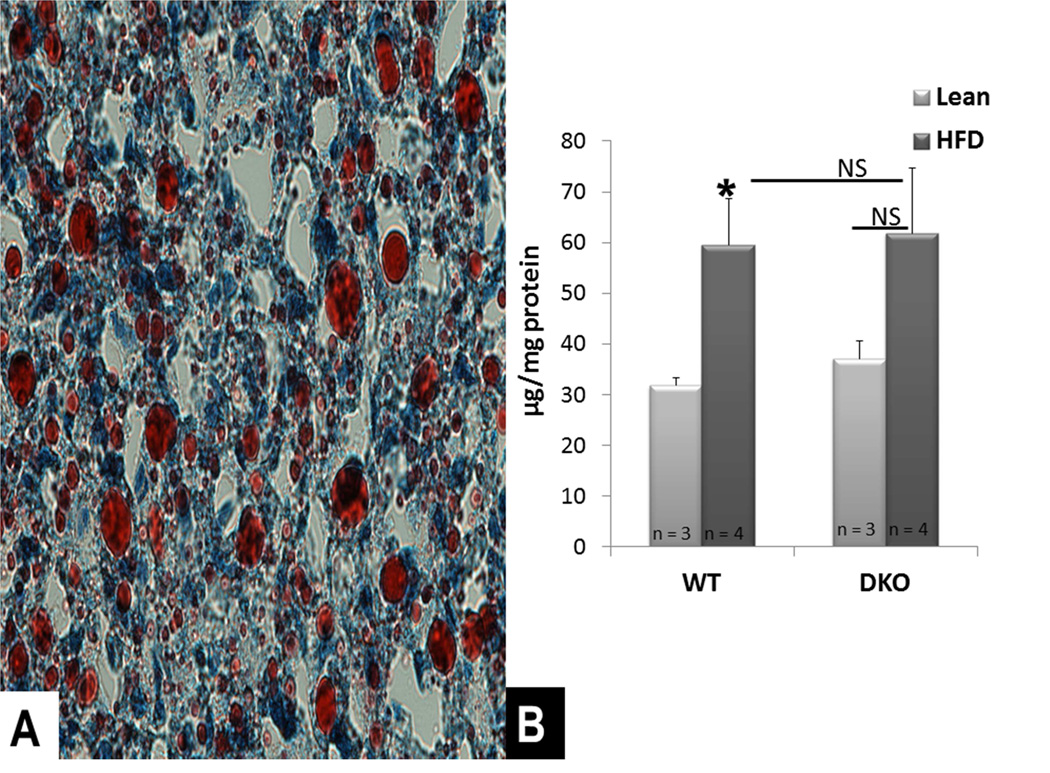
To investigate the role of BH3-only proteins in the development of hepatic steatosis, WT and DKO mice aged 9–11 weeks were fed HFD ad libitum. Following 5 weeks, each cohort had gained a significant amount of weight compared to their respective lean controls (p < 0.001 for both WT and DKO, figure 2). Oil red O staining demonstrated the development of steatosis in the WT liver (figure 3A). Both the WT and DKO had greater quantities of hepatic triglyceride following HFD compared to standard chow (figure 3B). There was no difference in the quantity of hepatic triglycerides between WT and DKO mice fed HFD (p = 0.89, figure 3B).
Figure 2. Hepatic Steatosis Induced through Dietary Modification is Not Affected by the Absence of BH3-only Proteins.
Following 5 weeks of HFD, each cohort gained a significant amount of weight and had increased levels of hepatic TG content compared to their respective lean controls.
Figure 3. Diet-induced hepatic steatosis.
A: Oil Red O staining for triglycerides shows development of macrosteatosis in WT mice fed HFD for 5 weeks (Image at 40x magnification). B: Both WT and DKO cohorts had greater quantities of hepatic triglyceride following HFD compared to standard chow. There was no difference in the quantity of hepatic triglycerides between WT and DKO mice fed HFD.
3.3 Absence of BH3-only proteins mitigates accentuation of IRI in the setting of steatosis
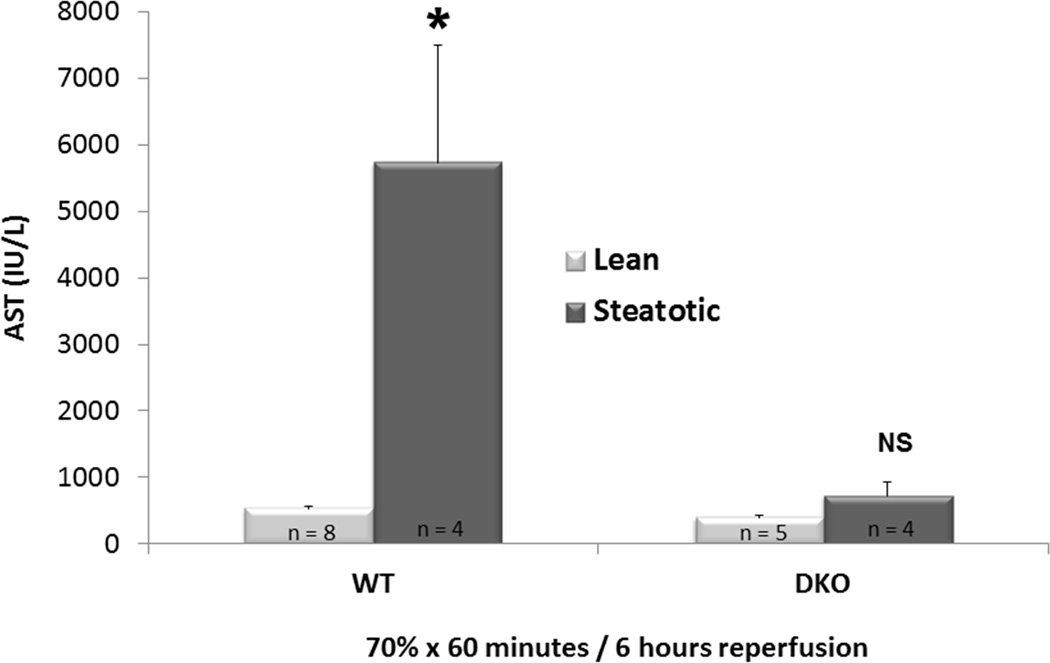
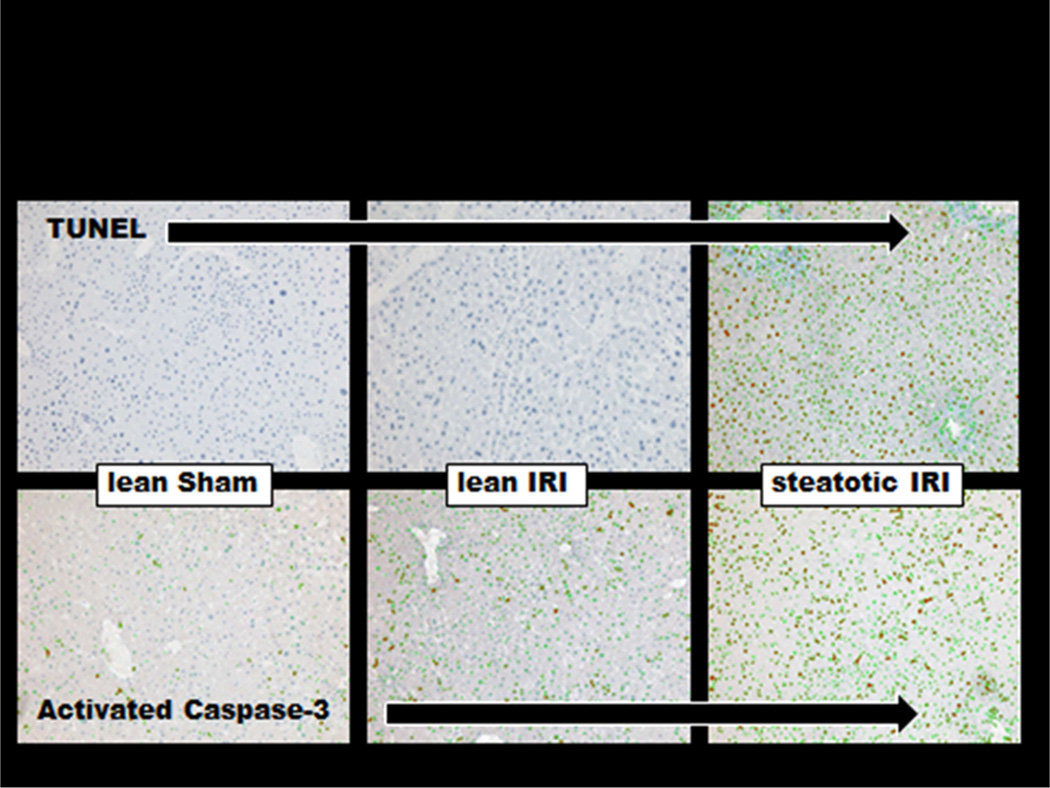
In WT mice fed HFD who underwent 70% hepatic ischemia for 60 minutes and 6 hours of reperfusion, there was a significantly greater injury compared to mice fed standard chow. (AST: 5728.8 IU/L ± 1776.9 vs. 524.9 IU/L ± 40.010, p < 0.01, figure 4). Increased staining for TUNEL and Caspase-3 showed that apoptosis played a critical role in this injury (figure 5).
Figure 4. Absence of Bim/Bid Proteins Mitigates Accentuation of IRI in Steatosis.
Steatosis accentuates IRI in WT mice fed HFD in comparison to their lean counterparts. In absence of Bim/Bid Proteins, steatosis did not complicate IRI. DKO mice fed HFD experienced IRI comparable to their lean counterparts.
Figure 5. IRI Complicated by Steatosis Induces Apoptosis.
IHC performed to assess the degree of apoptosis among lean and steatotic mice demonstrated apoptosis in the lean IRI, which was considerably greater in the setting of steatosis.
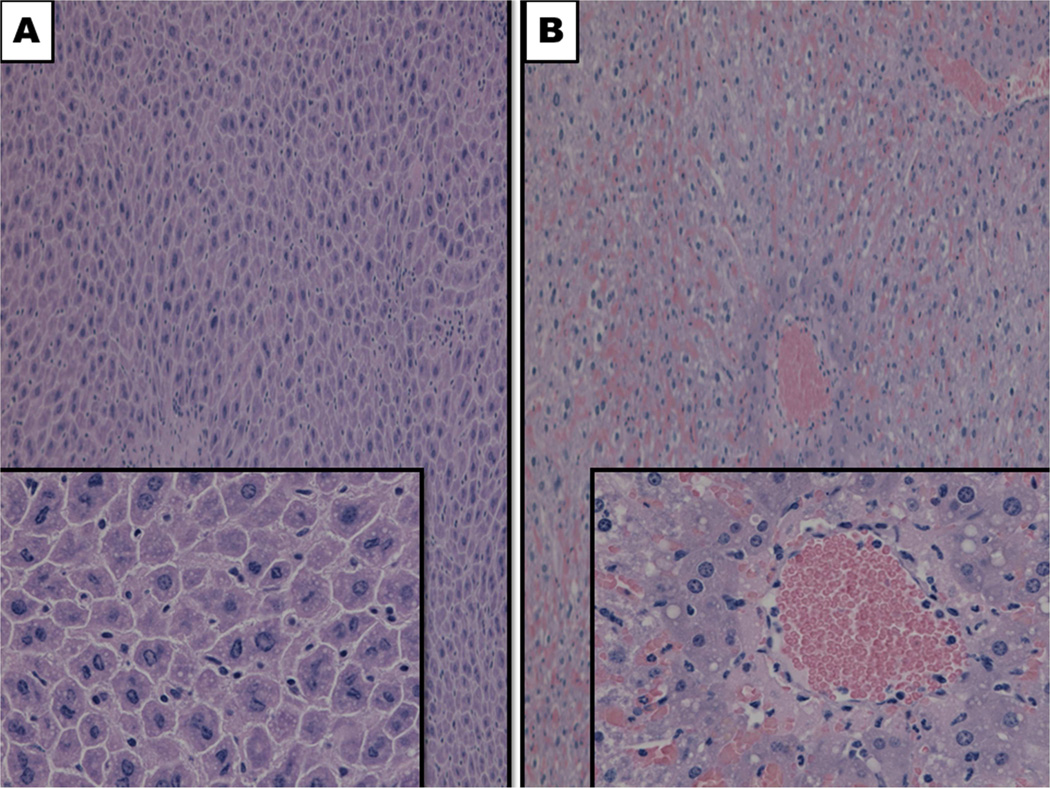
The DKO animals were protected from IRI despite equivalent hepatic steatosis relative to their WT counterparts (figure 4). The steatotic DKO liver did not have a significant increase in injury following ischemia and reperfusion compared to the lean DKO liver (AST: 393.2 ± 34.2 U/L vs. 717 ± 212.6 U/L, p = 0.13, figure 4). Histologic support for this finding was observed via the preservation of cytoarchitecture in steatotic DKO liver compared to their WT counterpart (figure 6).
Figure 6. BH3-only proteins are deleterious in IRI complicated by steatosis.
Relative preservation of hepatic cytoarchitecture was observed in DKO livers (A) following IRI compared to WT livers, which displaced considerable amounts of hepatic necrosis, congestion and vacuolization. Magnification 10x, 40x (insets).
4.0 DISCUSSION
Apoptosis is an important mediator of hepatocellular death from IRI, which is regulated through the actions of BH3-only protein members [5, 6]. While others have shown the importance of these BH3-only proteins in sepsis, their relevance in hepatic IRI, especially with steatosis, has been largely unknown [12]. This study aimed to investigate the role of BH3-only proteins, specifically Bim and Bid, in IRI of the steatotic liver.
In lean mice, the absence of Bim and Bid significantly attenuates hepatic IRI relative to WT controls. This was not a surprising finding. However, given the difference in cell death pathways that affect the steatotic liver, our study aimed to determine the role of Bim and Bid in steatosis IRI. We utilized a model of diet-induced hepatic steatosis, which parallels several features of steatosis in humans seen clinically. The DKO animals had weight gain and developed hepatic steatosis similarly to the WT animal which validates the comparisons of IRI between the steatotic cohorts. As others have shown, we demonstrated that hepatic steatosis increased the susceptibility of the liver to IRI. Lean, WT mice showed significantly less IRI than their steatotic counterparts. However, the same accentuation of IRI in steatosis was not appreciated in DKO mice. IRI in steatotic DKO mice was mitigated to levels comparable with their lean counterparts, suggesting a significant role of Bim and Bid in IRI in the steatotic liver.
Apoptosis has not been traditionally considered a major contributor to IRI injury in the steatotic liver. Our understanding of the multiple ways in which injured cells die has expanded such that it is now recognized that there is significant overlap in apoptosis, necrosis, and autophagy [17, 18]. Hepatocellular death in the steatotic liver has multiple initiating and modulating factors [17, 18]. This study suggests that major modulators of these factors are the BH3 proteins. A weakness of our study is the inability to determine the individual contribution to hepatic IRI by Bim or Bid. However, their importance in steatotic hepatic IRI injury was clear.
Bim and Bid are mediators of type II apoptosis in which is marked by mitochondrial depolarization and cytochrome c release. These BH3-only proteins are diverse in their activation mechanisms. Bid is cleaved by caspase 8 to its active truncated form. Bim is activated via c-Jun N-terminal Kinase (JNK) mediated phosphorylation [19]. Transcriptional control of Bim is regulated in part via mechanisms downstream of JNK and C/EBP homologous protein (CHOP) [19]. Our group and others have previously described the involvement of NFkB and ER stress mediators in steatotic liver injury [3, 20]. Based on with the findings of this study, Bim and Bid seem candidate downstream effectors of these previously described mediators.
Although further study is needed to describe exact mechanisms, our results demonstrate that the BH3 proteins play an important role in steatotic liver injury. This insight suggests that Bim and Bid may be potential therapeutic targets for the treatment or prevention of liver IRI.
Acknowledgments
Funding Sources: This work was supported in part by the American Society of Transplant Surgeons-Astellas Faculty Development Award (CDA), and National Institute of Health Grants: P30 DK056341, L30 DK082350 and R21 DK090661 (CDA)
Abbreviations
- IRI
ischemia-reperfusion injury
- BCL-2
B-cell lymphoma-2
- BH
BCL-2 Homology
- WT
wild type
- DKO
double knock out
- HFD
high fat diet
- TG
triglyceride
- TNF-α
tumor necrosis factor-alpha
- IL-1β
interleukin-1 beta
- DNA
deoxyribose nucleic acid
- siRNA
small interfering ribonucleic acid
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- TUNNEL
terminal deoxynucleotidyl transferase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presentation: Selected for presentation at the Association for Academic Surgeons / Society of University Surgeons 7th Annual Academic Surgical Congress. February 14th–16th, 2012. Las Vegas, NV.
Author Contributions: DuBray- Experimental concept, design, conduction, data collection, analysis, interpretation, writing, editing; Conzen- Experimental concept, design, conduction, data collection, review; Uphadaya- Experimental concept, design, editing, review; Gunter-Experimental conduction, data collection, review; Jia- Experimental concept, review; Knolhoff-Experimental conduction, data collection, review; Mohanakumar- Experimental concept, design, critique, editing; Chapman- Experimental concept, design, critique; Anderson- Experimental concept, design, interpretation, critique, writing, editing
Disclosure Statement: none
References
- 1.Ramalho FS, Fernandez-Monteiro I, Rosello-Catafau J, Peralta C. Hepatic microcirculatory failure. Acta Cir Bras. 2006;21(Suppl 1):48–53. doi: 10.1590/s0102-86502006000700012. [DOI] [PubMed] [Google Scholar]
- 2.Teramoto K, Bowers JL, Kruskal JB, Clouse ME. Hepatic microcirculatory changes after reperfusion in fatty and normal liver transplantation in the rat. Transplantation. 1993;56(5):1076–1082. doi: 10.1097/00007890-199311000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Anderson CD, Upadhya G, Conzen KD, Jia J, Brunt EM, Tiriveedhi V, Xie Y, Ramachandran S, Mohanakumar T, Davidson NO, et al. Endoplasmic reticulum stress is a mediator of posttransplant injury in severely steatotic liver allografts. Liver Transpl. 2011;17(2):189–200. doi: 10.1002/lt.22220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schröder M. Endoplasmic reticulum stress responses. Cell Mol Life Sci. 2008;65(6):862–894. doi: 10.1007/s00018-007-7383-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brenner D, Mak TW. Mitochondrial cell death effectors. Curr Opin Cell Biol. 2009;21(6):871–877. doi: 10.1016/j.ceb.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Montalvo-Jave EE, Escalante-Tattersfield T, Ortega-Salgado JA, Pina E, Geller DA. Factors in the pathophysiology of the liver ischemia-reperfusion injury. J Surg Res. 2008;147(1):153–159. doi: 10.1016/j.jss.2007.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Selzner N, Rudiger H, Graf R, Clavien PA. Protective strategies against ischemic injury of the liver. Gastroenterology. 2003;125(3):917–936. doi: 10.1016/s0016-5085(03)01048-5. [DOI] [PubMed] [Google Scholar]
- 8.Cheng EH, Wei MC, Weiler S, Flavell RA, Mak TW, Lindsten T, Korsmeyer SJ. BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol Cell. 2001;8(3):705–711. doi: 10.1016/s1097-2765(01)00320-3. [DOI] [PubMed] [Google Scholar]
- 9.Zong WX, Lindsten T, Ross AJ, MacGregor GR, Thompson CB. BH3-only proteins that bind pro-survival Bcl-2 family members fail to induce apoptosis in the absence of Bax and Bak. Genes Dev. 2001;15(12):1481–1486. doi: 10.1101/gad.897601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weber SU, Schewe JC, Lehmann LE, Müller S, Book M, Klaschik S, Hoeft A, Stüber F. Induction of Bim and Bid gene expression during accelerated apoptosis in severe sepsis. Crit Care. 2008;12(5):R128. doi: 10.1186/cc7088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang KC, Unsinger J, Davis CG, Schwulst SJ, Muenzer JT, Strasser A, Hotchkiss RS. Multiple triggers of cell death in sepsis: death receptor and mitochondrial-mediated apoptosis. FASEB J. 2007;21(3):708–719. doi: 10.1096/fj.06-6805com. [DOI] [PubMed] [Google Scholar]
- 12.Schwulst SJ, Muenzer JT, Peck-Palmer OM, Chang KC, Davis CG, McDonough JS, Osborne DF, Walton AH, Unsinger J, McDunn JE, et al. Bim siRNA decreases lymphocyte apoptosis and improves survival in sepsis. Shock. 2008;30(2):127–134. doi: 10.1097/shk.0b013e318162cf17. [DOI] [PubMed] [Google Scholar]
- 13.Institute of Laboratory Animal Resources (U.S.) NIH publication. Bethesda, Md.: U.S. Dept. of Health and Human Services; Committee on Care and Use of Laboratory Animals.: Guide for the care and use of laboratory animals. Public Health Service: v. [Google Scholar]
- 14.Kanoria S, Glantzounis G, Jalan R, Davies NA, Seifalian AM, Williams R, Davidson BR. A model to study total hepatic ischemia-reperfusion injury. Transplant Proc. 2004;36(9):2586–2589. doi: 10.1016/j.transproceed.2004.10.031. [DOI] [PubMed] [Google Scholar]
- 15.Sankary HN, Yin DP, Chong AS, Ma LL, Blinder L, Shen JK, Foster P, Liu LP, Li C, Williams JW. The portosystemic shunt protects liver against ischemic reperfusion injury. Transplantation. 1999;68(7):958–963. doi: 10.1097/00007890-199910150-00010. [DOI] [PubMed] [Google Scholar]
- 16.Ellett JD, Evans ZP, Atkinson C, Schmidt MG, Schnellmann RG, Chavin KD. Toll-like receptor 4 is a key mediator of murine steatotic liver warm ischemia/reperfusion injury. Liver Transpl. 2009;15(9):1101–1109. doi: 10.1002/lt.21782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malhi H, Gores GJ, Lemasters JJ. Apoptosis and necrosis in the liver: a tale of two deaths? Hepatology. 2006;43(2 Suppl 1):S31–S44. doi: 10.1002/hep.21062. [DOI] [PubMed] [Google Scholar]
- 18.Hotchkiss RS, Strasser A, McDunn JE, Swanson PE. Cell death. N Engl J Med. 2009;361(16):1570–1583. doi: 10.1056/NEJMra0901217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Engel T, Plesnila N, Prehn JH, Henshall DC. In vivo contributions of BH3-only proteins to neuronal death following seizures, ischemia, and traumatic brain injury. J Cereb Blood Flow Metab. 2011;31(5):1196–1210. doi: 10.1038/jcbfm.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tiriveedhi V, Conzen KD, Liaw-Conlin J, Upadhya G, Malone J, Townsend RR, Kerns R, Jia J, Csontos K, Ramachandran S, et al. The role of molecular chaperonins in warm ischemia and reperfusion injury in the steatotic liver: a proteomic study. BMC biochemistry. 2012:13–17. doi: 10.1186/1471-2091-13-17. [DOI] [PMC free article] [PubMed] [Google Scholar]