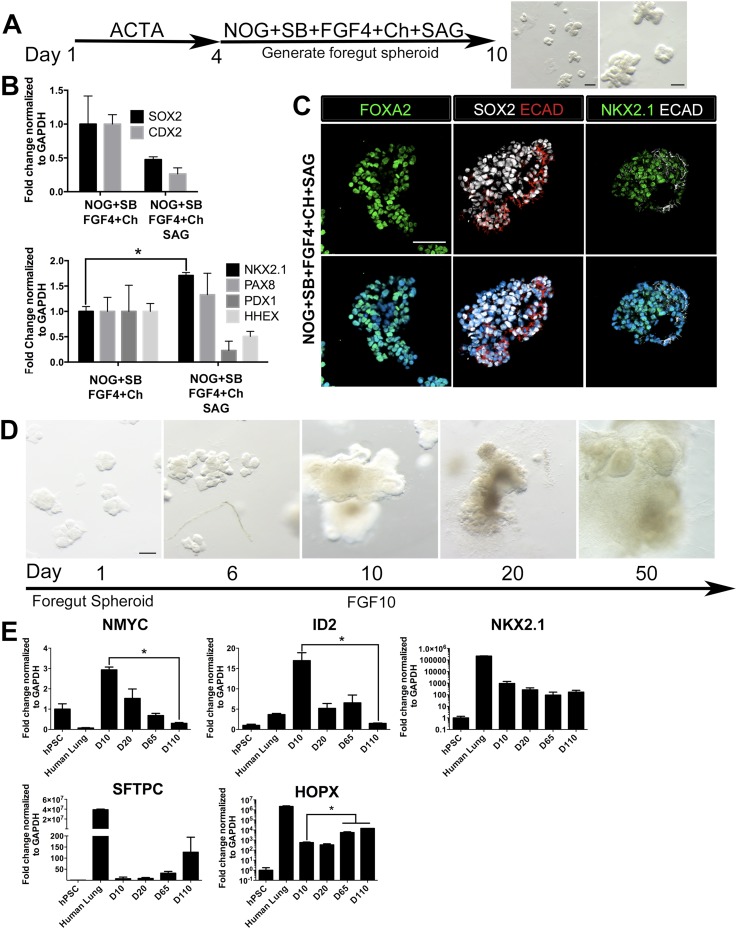
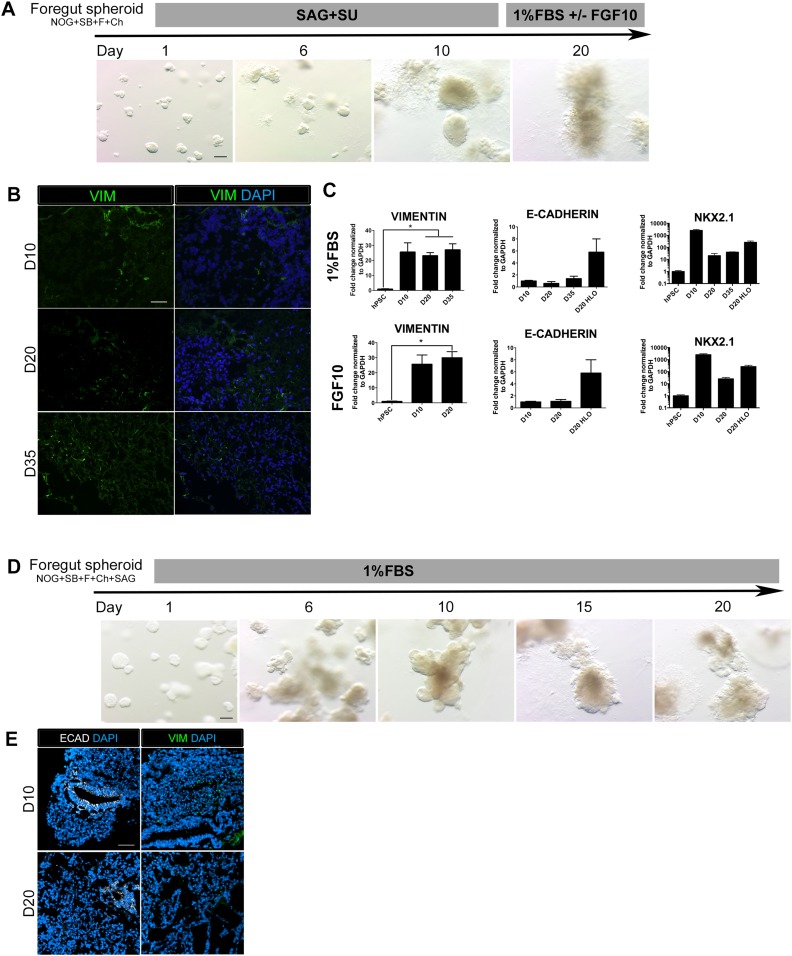
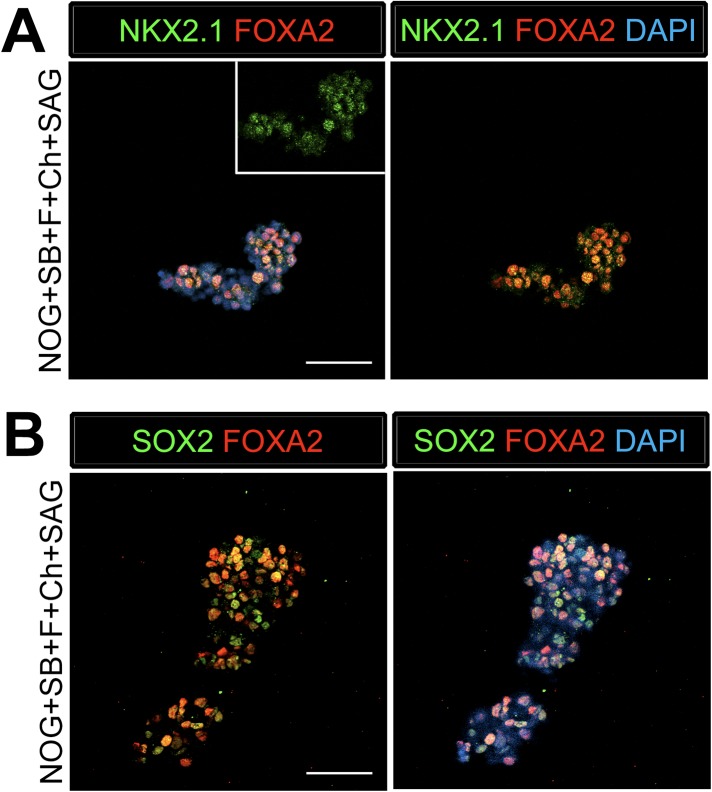
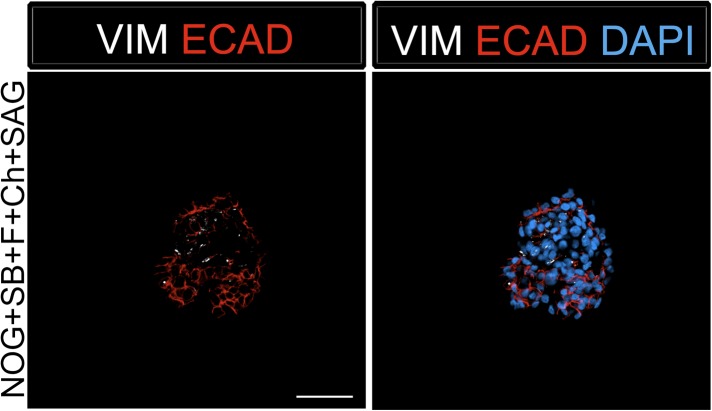
Figure 3. HH-induced ventral foregut spheroids give rise to lung organoids.
(A) hESCs were differentiated into foregut spheroids by treating cells with 4 days of ACTA and then an additional 4–6 days of NOG+SB+FGF4+Ch with the addition of the HH agonist SAG. Representative whole mount images of spheroids in a matrigel droplet are shown at low (left, scale bar 200 µm) and high magnification (right, scale bar 100 µm). (B) The addition of SAG to the NOG+SB+FGF4+Ch spheres caused a reduction in SOX2 and CDX2 transcripts (top panel) and a significant increase of NKX2.1 transcript (bottom panel) compared to NOG+SB+FGF4+Ch spheres (without SAG). Other foregut lineages (PAX8, PDX1, HHEX) were not significantly different when SAG was added. (C) The majority of the cells in NOG+SB+FGF4+Ch+SAG spheres expressed FOXA2, SOX2 and NKX2.1 protein. Scale bars represent 50 µm. (D) Timeline showing NOG+SB+FGF4+Ch+SAG induced foregut spheroids grown and maintained in FGF10. Note that Day 1 is the day spheroids were plated in Matrigel. The scale bar represents 100 µm. (E) Organoids express lung markers in a manner consistent with mouse lung development. All expression is shown relative to undifferentiated pluripotent stem cells (hPSC), and adult human lung is shown as a reference. Lung progenitor markers NMYC and ID2 were very low in adult lung, and were expressed at high levels in early organoid cultures, but were reduced over time (D = Days in culture), whereas NKX2.1 expression remained relatively constant. In contrast, SFTPC is known to be expressed at low levels in distal lung progenitors, but increases and is highly expressed in AECII cells. Consistently, SFTPC is highly expressed in adult human lungs and increases over time in organoid cultures and the AECI marker HOPX is also highly expressed in adult human lung and increases over time in organoids. *p < 0.05. All error bars represent SEM.