Abstract
Unicellular green algae of the genus Interfilum (Klebsormidiales, Streptophyta) are typical components of biological soil crusts. Four different aeroterrestrial Interfilum strains that have previously been molecular-taxonomically characterized and isolated from temperate soils in Belgium, Czech Republic, New Zealand, and Ukraine were investigated. Photosynthetic performance was evaluated under different controlled abiotic conditions, including dehydration, as well as under a light and temperature gradient. For standardized desiccation experiments, a new methodological approach with silica gel filled polystyrol boxes and effective quantum yield measurements from the outside were successfully applied. All Interfilum isolates showed a decrease and inhibition of the effective quantum yield under this treatment, however with different kinetics. While the single cell strains exhibited relatively fast inhibition, the cell packet forming isolates dried slower. Most strains fully recovered effective quantum yield after rehydration. All Interfilum isolates exhibited optimum photosynthesis at low photon fluence rates, but with no indication of photoinhibition under high light conditions suggesting flexible acclimation mechanisms of the photosynthetic machinery. Photosynthesis under lower temperatures was generally more active than respiration, while the opposite was true for higher temperatures. The presented data provide an explanation for the regular occurrence of Interfilum species in soil habitats where environmental factors can be particularly harsh.
Keywords: aeroterrestrial algae, biological soil crust, ecophysiology, photosynthesis, respiration
While many green algae occur under freshwater and marine conditions, they are not restricted to these aquatic systems. Green algae may also be found in diverse types of terrestrial environments, such as on and under rocks, on plant surfaces or in association with soils (Ettl and Gärtner 1995). Only hydrated cells will be physiologically active (Häubner et al. 2006, Gray et al. 2007, Karsten et al. 2007). In aquatic ecosystems, most algae are only rarely confronted with dehydration and are potentially capable of being continuously metabolically active. In contrast, aeroterrestrial algae often experience water loss, and hence they can be metabolically active only when there is sufficient water supply. Dehydration strongly affects photosynthesis and growth and has destructive effects on the ultrastructure of the cells (Holzinger and Karsten 2013). In temperate regions, water supply changes with the course of the seasons from humid periods with rain, dew, or snow to extended periods of drought or freezing. Water supply is therefore the key ecological factor for long-term survival of aeroterrestrial green algae (Holzinger and Karsten 2013). Besides the effects by fluctuating meteorological conditions on the water status of the cell, intrinsic factors like cell wall properties, extracellular polymeric substances (EPS), formation of cell aggregates, or the biochemical capability to synthesize osmotically active substances represent an important mechanism for algal cells to hold water (Yancey 2005, Flechtner 2007, Rindi 2007). In addition, aeroterrestrial algae are often exposed to other harsh environmental conditions in comparison to their aquatic relatives, such as fluctuating temperatures and high insolation (photosynthetic active radiation [PAR] and UV; Gray et al. 2007). Therefore, aeroterrestrial algae have to be well adapted to these often extreme physical factors to guarantee long-term survival in terrestrial environments.
Recently, the evolution and phylogeny of green algae was critically reviewed by Leliaert et al. (2012 and references therein). These authors showed that the Viridiplantae is comprised of two discrete lineages, the Chlorophyta and Streptophyta. The Chlorophyta consists of several classes including the Trebouxiophyceae, which contains many aeroterrestrial taxa (Friedl and Rybalka 2012). The other lineage, the Streptophyta, includes the charophytes from which the land plants evolved, and which is considered as an older phylum compared to land plants (Leliaert et al. 2012). Typical aeroterrestrial representatives of the streptophytan lineage are members of the Klebsormidiophyceae, that include the genera Klebsormidium and Interfilum, which form unbranched filaments, unicellular aggregations, cell packets, or sarcinoid colonies (Mikhailyuk et al. 2008, Sluiman et al. 2008, Rindi et al. 2011).
Interfilum R.Chodat species are considered important and widely distributed components in various terrestrial habitats, particularly on soils or in association with biological soil crusts (Mikhailyuk et al. 2008, Rindi et al. 2011, and references therein). Many reports of their distribution suggest that they originated from Europe, but Interfilum has also been described in recent years from Argentina (Ehrenhaus and Vigna 2008), New Zealand (Novis and Visnovsky 2011), and Antarctica (Worland and Lukešová 2000), indicating that members of this genus have probably a worldwide distribution similar to the closely related Klebsormidium (Rindi et al. 2011). Molecular data of the New Zealand isolate of Interfilum terricola (Novis and Visnovsky 2011) are in close agreement with those of this species from Belgium (SAG 2100) (Mikhailyuk et al. 2008). Consequently, Novis and Visnovsky (2011) concluded that based on these genetic data, diversification has not occurred in isolation in New Zealand, and that global dispersal of Interfilum must occur quite readily, with no detectable difference from European strains. Closely related Klebsormidium strains can be successfully established as cultures from airborne-samples collected from aircraft in flight (Brown et al. 1964), supporting the concept of ease of dispersal. Sharma et al. (2007) indicate that typical aeroterrestrial green algal taxa such as Klebsormidium (and probably Interfilum) can be dispersed as single cells, filaments (or fragments of filaments), and/or spores via atmospheric transport, and hence it is reasonable to assume that colonization of new terrestrial surfaces such as soil is mediated by such airborne algal cells or spores. However, Interfilum has not yet been reported for many geographic regions. The reason could be simply related to the fact that the identification of unicellular and sarcinoid green algae generally presents a challenge due to the scarcity of visual characters that are available for diagnostic purposes (Mikhailyuk et al. 2008).
Along with other microorganisms such as bacteria, cyanobacteria, and fungi, as well as with macroscopic lichens and bryophytes, Interfilum represents an important phototrophic component of biological soil crusts (Mikhailyuk et al. 2008). These communities produce a joint matrix by gluing soil particles to themselves, thereby forming productive microbial biomasses in the “earth critical zone” which represents the uppermost ∼10 mm of soils in many drylands (Belnap and Lange 2001, Pointing and Belnap 2012). Such microbiotic crusts exert a dominating influence on global carbon fixation (∼7% of terrestrial vegetation) and nitrogen fixation (∼50% of terrestrial biological N fixation; Elbert et al. 2012), along with other ecological functions such as mineralization, water retention, stabilization of soils, and dust trapping (Evans and Johansen 1999, Reynolds et al. 2001, Lewis 2007, Castillo-Monroy et al. 2010).
Although the ecological roles of Klebsormidiales are considered significant, only a few studies exist on their ecophysiological response patterns as a function of environmental stress scenarios. While this lack of knowledge has been at least partly addressed in recent years for Klebsormidium from urban and alpine habitats (Karsten and Rindi 2010, Karsten et al. 2010, Holzinger et al. 2011, Kaplan et al. 2012, Karsten and Holzinger 2012), Interfilum is still completely unstudied.
In the present study, we examined for the first time the ecophysiological performance of four different strains of Interfilum from temperate soils in Belgium, Czech Republic, Ukraine, and New Zealand. These sites exhibit a gradient of precipitation ranging from 388 mm (Karadag, Crimea, Ukraine) to 1,162 mm annual rainfall (Waroneu, Belgium; http://www.worldclimate.com). According to Rindi et al. (2011) this genus forms the superclade A in his molecular-phylogenetic treatment of Klebsormidium/Interfilum, and the four investigated Interfilum isolates were assigned to subclade A1 (Interfilum massjukiae), A2 (I. terricola), A3 (Interfilum sp.), and A4 (Interfilum sp.). While I. massjukiae and I. terricola represent morphologically well described species, A3 and A4, the two earliest-diverging subclades, could not be identified unambiguously, and hence may represent undescribed taxa (Mikhailyuk et al. 2008, Rindi et al. 2011). The four Interfilum strains exhibited different morphologies ranging from single cells to cell packets in combination with the occurrence or lack of mucilage.
The physiological parameters (i.e., dehydration and temperature tolerance) and the light requirements for photosynthesis were investigated under the same conditions in the four Interfilum strains. The main goal of this study was to evaluate whether morphology and mucilage influence dehydration dynamics and the physiology of the organisms. Of particular interest was to consider whether the precipitation features of the original habitat are reflected in the respective response patterns.
Materials and Methods
Strain origin and culture conditions
The Interfilum strains SAG 2100 (I. terricola), SAG 2102 (I. massjukiae), SAG 36.88 (Interfilum sp.), and SAG 2147 (Interfilum sp.) were purchased from The Culture Collection of Algae at Göttingen University, Germany (international acronym SAG; http://www.uni-goettingen.de/en/184982.html). They represent some of the few available clonal cultures. All isolates deposited in the SAG were collected from soils in Europe or New Zealand. Information on origin and habitat, meteorological data, as well as taxonomic assignments are summarized in Table1.
Table 1.
Characterization of the Interfilum isolates collected from temperate soils
| Strain number | Habitat and origin | Meteorological data | Clade assignment according to Rindi et al. (2011) | Species assignment and authority; Sequence Accession (18s rRNA, ITS1, 5.8s rRNA, ITS2, 26s rRNA) |
|---|---|---|---|---|
| SAG 2100 | Soil, oak forest, Haute Ardenne, Belgium; 440 m above sea level, Waroneu experimental site (sampling point D°QL-120); isolated 1996 by I. Kostikov |
Min. air temperature: −3°C to 10°C Max. air temperature: 3°C–21°C Monthly rainfall days: 18–22 Monthly precipitation: 74–165 mm Annual rainfall: 1162 mm |
A2 | Interfilum terricola (B. Petersen) Mikhailyuk, Sluiman, Massalski, Mudimu, Demchenko, Friedl et Kondratyuk; EU434040 |
| SAG 2102 | Soil, surface of pyroclastic outcorps, Karadag nature reserve, Crimea, Ukraine; isolated April 2005 by E. Demchenko |
Min. air temperature: −1°C to 19°C Max. air temperature: 3°C–28°C Monthly rainfall days: 8–16 Monthly precipitation: 20–38 mm Annual rainfall: 388 mm |
A1 | Interfilum massjukiae Mikhailyuk, Sluiman, Massalski, Mudimu, Demchenko, Friedl et Kondratyuk; EU434038 |
| SAG 36.88 | Soil, Tekoa, New Zealand; isolated before 1988 by E.A. Flint |
Min. air temperature: 1°C–12°C Max. air temperature: 11°C–22°C Monthly rainfall days: 8–14 Monthly precipitation: 41–68 mm Annual rainfall: 615 mm |
A3 | Interfilum sp.; EU434027 |
| SAG 2147 | Soil, Ceske Stredohori Mountains, top of Borec Hill, Czech Republic; 508 mm annual rain fall; isolated January 2003 by P. Skaloud |
Min. air temperature: −3°C to 14°C Max. air temperature: 2°C–24°C Monthly rainfall days: 17–21 Monthly precipitation: 28–65 mm Annual rainfall: 508 mm |
A4 | Interfilum sp.; EU434039 |
Strain number, habitat and origin, taxonomic assignment according to the suggested clades of Rindi et al. (2011) and identification are given. The meteorological data include monthly minimum air temperature, monthly maximum air temperature, monthly rainfall days, monthly precipitation (mm), and annual precipitation (mm) (http://www.worldweatheronline.com). The sequence accession numbers include complete and partial sequences, respectively, of the different ribosomal regions. SAG: Sammlung für Algenkulturen, Göttingen, Germany.
For cultivation of the four Interfilum stock cultures, Erlenmeyer flasks (volume 250–500 mL) filled with modified Bold's Basal Medium (3NBBM; Starr and Zeikus 1993) were used. The same culture conditions and equipment was used as described for Klebsormidium (Karsten and Holzinger 2012), i.e., algae were kept at 20°C and 35–40 μmol photons · m−2 · s−1 under a light:dark cycle of 16:8 h L:D. As light sources, Osram Daylight Lumilux Cool White lamps (L36W/840; Osram, Munich, Germany) were used. Radiation measurements were carried out with a Solar Light PMA 2132 cosine corrected PAR sensor connected to a Solar Light PMA 2100 radiometer (Solar Light Co., Inc., Philadelphia, PA, USA). Log-phase cultures were used for all experiments, which was regularly verified by microscopic cell counts using a hemacytometer couting chamber according to Guillard and Sieracki (2005).
Light microscopy
For light microscopy, subsamples of log-phase cultures, taken at the beginning of the dehydration experiments, were used. Light microscopy was performed with a Zeiss Axiovert 200 M microscope, equipped with a 63× 1.4 NA objective lens. Images were generated by differential interference contrast and captured with an Axiocam MRc5 camera and Zeiss Axiovision (Carl Zeiss Microscopy GmbH, Jena, Germany) software. Images were further processed by Adobe Photoshop (Adobe Systems Inc., San José, CA, USA) (CS5) software version 12.1. For determination of the cell dimensions (cell width, cell length) a minimum of 10 cells were measured.
Dehydration experiments using pulse amplitude modulation fluorometry
For the desiccation experiments, a new standardized set-up was developed to follow the kinetics of controlled dehydration and subsequent rehydration on the effective quantum yield of photosystem II (PSII) using noninvasive pulse amplitude modulation (PAM) fluorometry. All PAM measurements were done on low-light acclimated samples (35–40 μmol photons · m−2 · s−1). In addition, the effect of increasing temperatures on photosynthesis and respiration was recorded using an oxygen optode.
Cells of each Interfilum strain were concentrated on four replicate Whatman GF/F glass fiber filters (Whatman, Dassel, Germany). Onto each filter, exactly 200 μL of the cell suspension (∼1–2 mg chl a · L−1; parallel filters for chl a concentration were determined using dimethyl formamide [DMF] as described below) was concentrated in the center as a light green spot using an Eppendorf Pipette. These moist filters were positioned on perforated metal grids (hole diameter: 1 mm; distance between holes: 1.5 mm) on top of four glass columns inside a transparent 200 mL polystyrol box, which was filled with 100 g of freshly activated silica gel (Silica Gel Orange, Carl Roth, Karlsruhe, Germany) and sealed with a transparent top lid (Fig.1). To record the relative air humidity (RAH) conditions inside the chambers, a PCE-MSR145S-TH mini data logger for air humidity and temperature was employed (PCE Instruments, Meschede, Germany; Fig.1). The boxes were kept under ambient room temperatures at 22°C ± 1°C and 40 μmol photons · m−2 · s−1 PAR (Osram light sources see above).
Figure 1.
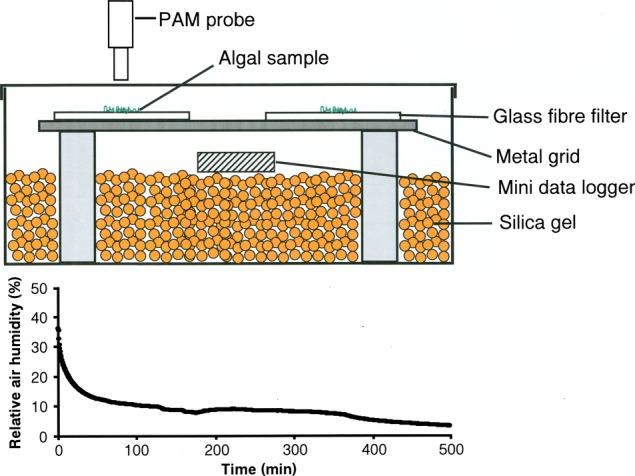
Above: Schematic diagram of the desiccation chamber. Moist glass fiber filters with algal cells on top were positioned on a perforated metal grid on top of four glass columns inside a transparent 200 mL polystyrol box, which was filled with 100 g silica gel and sealed with a transparent top lid. A mini data logger for air humidity and temperature was employed inside the boxes on top of the silica gel for continuous measurements. The PAM light probe was positioned outside the cover lid of the box (∼2 mm distance), so that all fluorescence measurements were done through the polystyrol lid. Below: Course of the relative air humidity (RAH) conditions inside the desiccation chambers after onset of an experiment. A PCE-MSR145S-TH mini data logger for air humidity and temperature was employed inside the boxes for continuous measurements (PCE Instruments).
The effective quantum yield (ΔF/Fm') of PSII was regularly determined during the dehydration period (350–470 min depending on the strain) using a pulse amplitude modulated fluorimeter (PAM 2500; Heinz Walz GmbH, Effeltrich, Germany) according to the approach of Genty et al. (1989). 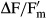 was calculated as
was calculated as 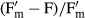 with F as the fluorescence yield of light-treated algal cells (40 μmol photons · m−2 · s−1) and
with F as the fluorescence yield of light-treated algal cells (40 μmol photons · m−2 · s−1) and 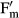 as the maximum light-adapted fluorescence yield after employing a 800 ms saturation pulse as described by Schreiber and Bilger (1993). The PAM light probe was positioned outside the cover lid of the boxes (always 2 mm distance) to guarantee undisturbed RAH conditions inside, i.e., all fluorescence measurements were done through the polystyrol lids (Fig.1). The distance from the PAM light probe to the algal sample onto the glass fiber filters was always kept constant at 10 mm.
as the maximum light-adapted fluorescence yield after employing a 800 ms saturation pulse as described by Schreiber and Bilger (1993). The PAM light probe was positioned outside the cover lid of the boxes (always 2 mm distance) to guarantee undisturbed RAH conditions inside, i.e., all fluorescence measurements were done through the polystyrol lids (Fig.1). The distance from the PAM light probe to the algal sample onto the glass fiber filters was always kept constant at 10 mm.
After the dehydration period, the dried glass fiber filters were transferred to a new polystyrol box which was filled with 100 mL tap water instead of silica gel to create a high humidity atmosphere (>95%). The filters were rehydrated by adding 200 μL of the standard growth medium to each filter and recovery of 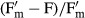 was followed with the same methodology as described (i.e., at 22°C ± 1°C and 40 μmol photons · m−2 · s−1).
was followed with the same methodology as described (i.e., at 22°C ± 1°C and 40 μmol photons · m−2 · s−1).
Light experiments using PAM fluorometry
Besides determination of the effective quantum yield, relative electron transport rates (rETR) under increasing photon fluence densities were followed in log-phase Interfilum samples. Since with the halogen lamp of the oxygen optode system only 500 μmol photons · m−2 · s−1 could be realized (see below), a PAM 2500 was additionally used to apply 3-fold higher irradiances for the determination of light-induced rETR and possible photoinhibitory effects.
Cells of each Interfilum strain were concentrated on four replicate Whatman GF/F glass fiber filters as previously described and positions in the polystyrol box filled with 200 mL tap water. These moist filters were also positioned in the transparent 200 mL, tap water filled polystyrol boxes, and treated as already described. Algal cells were exposed to 13 photon flux densities (PFDs) for 30 s each (rapid light curves) ranging from 1 up to 1,432 μmol photons · m−2 · s−1. The actinic light was provided by a red power LED (630 nm) of the PAM 2500. After each light exposure, a saturating pulse was given to detect Fm and 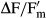 . The rETR of PSII was calculated according to Kromkamp and Forster (2003):
. The rETR of PSII was calculated according to Kromkamp and Forster (2003):
where 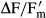 = the effective PSII quantum efficiency and PFD = photon flux density.
= the effective PSII quantum efficiency and PFD = photon flux density.
All measurements were undertaken under ambient room temperatures at 22°C ± 1°C. Photosynthesis-irradiance (PI) curves as rETR versus PFD were calculated to check whether the four strains of Interfilum show indications of photoinhibition. The data were fitted by the mathematical photosynthesis model of Webb et al. (1974) without photoinhibition.
Photosynthesis and respiration measurements using an oxygen optode
For measuring photosynthetic oxygen production rates under increasing photon fluence densities (PI curves) and respiratory oxygen consumption in the dark, a Presens Fibox 3 oxygen optode (Presens, Regensburg, Germany) was used. The oxygen sensor was attached to a 3 mL thermostatic acrylic chamber (type DW1, Hansatech Instruments, Norfolk, UK) combined with a magnetic stirrer according to Remias et al. (2010). Samples of 2.8 mL log-phase algal suspension were added to the chamber as well as 0.2 mL NaHCO3 stock solution (resulting in a final concentration of 2 mM NaHCO3) to ensure sufficient carbon supply during incubation. Algal cells were exposed to nine photon fluence rates ranging from 0 to 500 μmol photons · m−2 · s−1 PAR (photosynthetically active radiation for 10 min) as described in Karsten and Holzinger (2012). In addition, the respiration rate was measured in darkness directly before the onset of the increasing photon fluence rates (each stage 10 min) as well as directly after offset of the highest light level. The O2 production per photon fluence density and time was normalized to the amount of total chl a per sample. To measure chl a, after each PI curve measurement, the cell suspension was filtered onto a Whatman GF/F glass fiber filter using a glass Pasteur pipette. Algal cell chl a was extracted with 3 mL DMF and photometrically quantified according to Porra et al. (1989). PI curve data were calculated and fitted again by the mathematical photosynthesis model of Webb et al. (1974), which allowed the calculation of the three characteristic parameters: α, positive slope at limiting photon fluence rates (μmol O2 · h−1. mg−1 Chl a · (μmol photons−1 · m−2. s-1)-1); Ic, light compensation point (μmol photons · m−2 · s−1); Ik, initial value of light-saturated photosynthesis (μmol photons · m−2 · s−1).
To examine the effect of rising temperatures on photosynthetic and respiratory response patterns in the four Interfilum strains, a Thermo Haake K20 refrigerated circulator (Thermo Fisher Scientific Inc., Waltham, MA, USA) was connected to the measuring chamber. The algal samples were exposed to nine temperature steps ranging from 5°C to 45°C in 5°C increments, similar to the experimental design of Karsten and Holzinger (2012). The O2 consumption and production per time unit was referenced to the concentration of total chl a per sample as described above.
From the final photosynthetic and respiratory rates (μmol O2 · mg−1 · Chl a · h−1), the gross photosynthesis:respiration (P:R) ratios for each temperature were calculated.
Statistical analysis
All PAM experiments were carried out with four independent replicates, while all optode experiments had three. The shown data represent the respective mean values ± SD.
Statistical significance of the means of the effective quantum yield of dehydrated and rehydrated samples, of rETR values, as well as of the optode data were tested with one-way ANOVA followed by a Tukey's multiple comparison test, to find subgroups of means with significant differences. Analyses were performed with InStat (GraphPad Software Inc., La Jolla, CA, USA).
Results
Light microscopy
I. massjukiae SAG 2102 occurred either unicellularly or formed cell packages consisting of 2–4 cells (Fig.2, A and B). Occasionally uni- and biserate branched filaments were observed (Fig.2B). This strain had thick cell walls, but lacked a mucilage envelope (Fig.2, A and B). The formation of filaments or aggregates is due to incorporating parts of the detaching mother cell wall in the growing daughter cell walls resulting in a sarcinoid growth. Individual cells were mostly ellipsoid and had a cell width of 4.9–6.5 μm. However, when cells were part of an aggregate, they became hemispherical or rectangular and increased in size (Fig.2A). The single parietal chloroplast was dissected in several lobes and contained one pyrenoid surrounded by starch grains (Fig.2A). A similar chloroplast shape was observed in I. terricola SAG 2100 (Fig.2, C and D). However, these cells were surrounded by a mucilage layer and only rarely formed aggregates (Fig.2C). Most cells were ellipsoid with a cell width between 4.7 and 5.4 μm. Remains of the mother cell wall attached to the descendent cell wall were observed frequently (Fig.2D). This phenomenon was occasionally also seen in Interfilum sp. SAG 36.88 (Fig.2E). Moreover, Interfilum sp. SAG 36.88 had a mucilage envelope and a parietal chloroplast with one central pyrenoid (Fig.2E). Strain Interfilum sp. SAG 36.66 rarely formed aggregates, but had distinct stretched cylindrical cells with a length of 6.5–7.9 μm and an average width of 3.7 μm, resulting in a length to width ratio of ∼2 (Fig.2E). This is in contrast to the observations in strain I. massjukiae SAG 2102 (Fig.2, A and B) and I. terricola SAG 2100 (Fig.2, C and D), which had more ellipsoid cells. The cells of strain Interfilum sp. SAG 2147 were aggregated in conspicuous sarcinoid packages of five and more cells, and no mucilage coated the thick cell wall (Fig.2F). The chloroplast morphology was similar to the other strains. The cell width of Interfilum sp. SAG 2147 ranged from 4.6 to 5.5 μm (Fig.2F).
Figure 2.
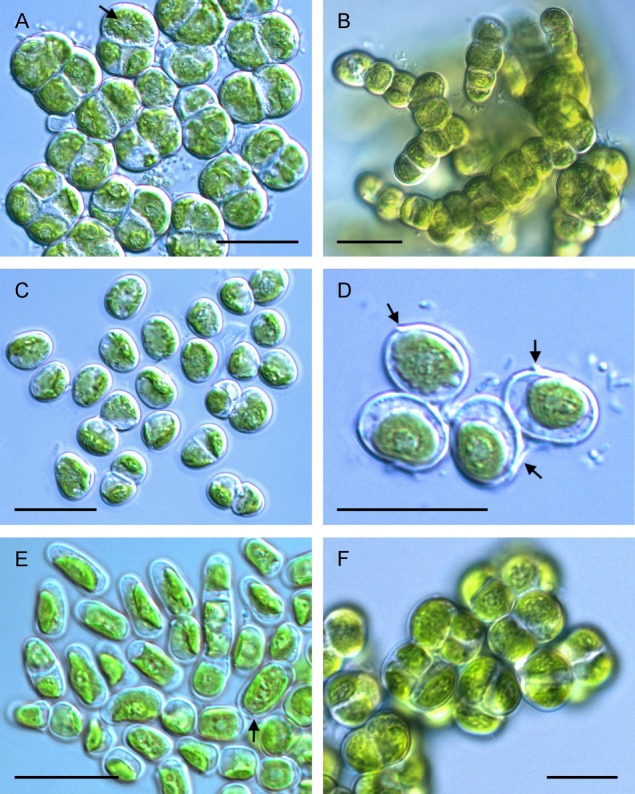
Morphology of four strains of Interfilum (Klebsormidiales, Streptophyta) belonging to the superclade A (Rindi et al. 2011). (A) Cell packets of Interfilum massjukiae SAG2102 (clade A1) with the central pyrenoid surrounded by numerous starch grains (arrow), (B) sarcinoid aggregations and uniserate filaments in the same culture, (C) Interfilum terricola SAG2100 (clade A2), (D) SAG2100 with remains of the mother cell wall (arrows). The lobed chloroplast is clearly visible in the upper left cell, (E) stretched cells of Interfilum sp. SAG36.88 (clade A3) with remains of mother cell walls (arrow), (F) Interfilum sp. SAG2147 (clade A4) forming sarcinoid cell packages; scale bars = 10 μm.
Dehydration and rehydration
Using the new methodological approach with the silica gel filled polystyrol boxes and PAM measurements from the outside (Fig.1), it was possible to undertake standardized comparative effective quantum yield determinations on all Interfilum strains during the dehydration and rehydration experiments (Figs.3 and 4). Figure1 shows the typical RAH conditions inside the boxes during the course of a desiccation experiment. Within the first 30 min, the RAH declines from ∼30% to 10% and remains after that almost unchanged at this value (or slightly below), which indicates a reproducible dry atmosphere. There were conspicuous differences in the effective quantum yield among the four Interfilum strains during desiccation. While I. terricola SAG 2100 showed a continuous decrease of 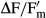 over a period of 400 min down to a minimum signal of 20% of the control, all other isolates exhibited an unchanged maximum effective quantum yield for some time before a threshold was reached, after which the fluorescence signals strongly dropped to less than 10% of the control (Fig.3). The interval of unaffected
over a period of 400 min down to a minimum signal of 20% of the control, all other isolates exhibited an unchanged maximum effective quantum yield for some time before a threshold was reached, after which the fluorescence signals strongly dropped to less than 10% of the control (Fig.3). The interval of unaffected 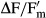 was 210 min in Interfilum sp. SAG 36.88, 240 min in I. massjukiae SAG 2102, and 310 min in Interfilum sp. SAG 2147. After these periods, the effective quantum yield decreased within 90 min to 0%–10% of the control in I. massjukiae SAG 2102 and Interfilum sp. SAG 2147, while it took 195 min in Interfilum sp. SAG 36.88 (Fig.3). When I. terricola SAG 2100 was dried for a much longer time (800–1,000 min), the fluorescence values further decreased to less than 10% of the control (data not shown).
was 210 min in Interfilum sp. SAG 36.88, 240 min in I. massjukiae SAG 2102, and 310 min in Interfilum sp. SAG 2147. After these periods, the effective quantum yield decreased within 90 min to 0%–10% of the control in I. massjukiae SAG 2102 and Interfilum sp. SAG 2147, while it took 195 min in Interfilum sp. SAG 36.88 (Fig.3). When I. terricola SAG 2100 was dried for a much longer time (800–1,000 min), the fluorescence values further decreased to less than 10% of the control (data not shown).
Figure 3.
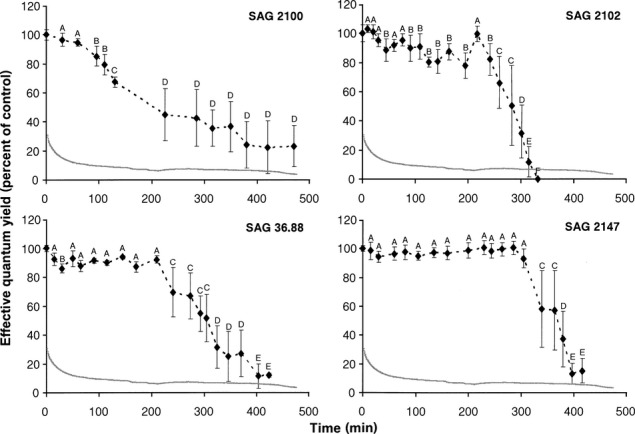
The effect of controlled desiccation on the effective quantum yield (ΔF/Fm') of photosystem II as regularly measured with a PAM 2500 during the experiment (450–500 min) in the four strains of Interfilum (n = 4, mean value ± SD). SAG2100: Interfilum terricola; SAG2102: Interfilum massjukiae; SAG36.88: Interfilum sp.; SAG2147: Interfilum sp. Effective quantum yield values of control algae under 40 μmol photons · m−2 · s−1 PAR was determined as 0.41–0.52 and standardized to 100% for better comparison. All measurements were done at 22°C ± 1°C. The gray lines show the course of the relative air humidity between 30% and 10% conditions inside the desiccation chambers after onset of an experiment (see Fig.1). Significances among the treatments were calculated by one-way ANOVA (I. terricola SAG 2100, F12,39 = 40.8, P < 0.01; I. massjukiae SAG 2102, F18,57 = 164.4, P < 0.01; Interfilum sp. SAG 36.88, F17,54 = 132.5, P < 0.05; Interfilum sp. SAG 2147, F17,54 = 95.3, P < 0.05). Different capital letters represent significant differences among the time points as revealed by Tukey's post hoc test.
Figure 4.
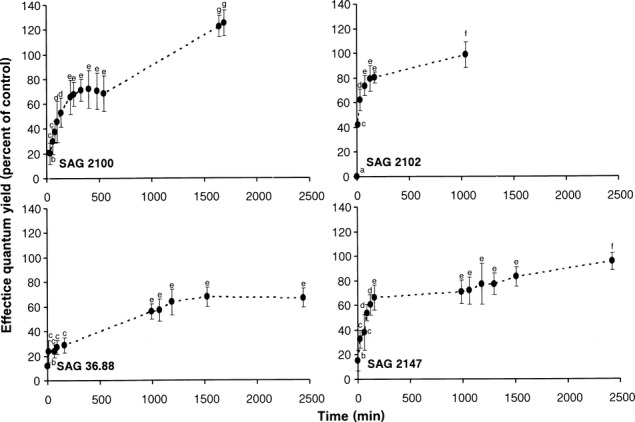
The effect of controlled rehydration on the effective quantum yield (ΔF/Fm') of photosystem II in the desiccated algal samples (see Fig.3) as measured with a PAM 2500 during the experiment (1,000–2,450 min depending on the response) in the four studied strains of Interfilum (n = 4, mean value ± SD). SAG2100: Interfilum terricola; SAG2102: Interfilum massjukiae; SAG36.88: Interfilum sp.; SAG2147: Interfilum sp. Effective quantum yield values of control algae (see Fig.3) under 40 μmol photons · m−2 · s−1 PAR was determined as 0.41–0.52 and standardized to 100% for better comparison. All measurements were done at 22°C ± 1°C. Significances among the treatments were calculated by one-way ANOVA (I. terricola SAG 2100, F15,48 = 151.9, P < 0.05; I. massjukiae SAG 2102, F9,30 = 135.9, P < 0.01; Interfilum sp. SAG 36.88, F9,30 = 105.6, P < 0.05; Interfilum sp. SAG 2147 − F9,30 = 91.4, P < 0.05). Different small letters represent significant differences among the time points as revealed by Tukey's post hoc test.
After dehydration, all Interfilum samples were rehydrated. While I. terricola SAG 2100, I. massjukiae SAG 2102, and Interfilum sp. SAG 2147 reached 70%–80% of the control effective quantum yield within 230 min, in Interfilum sp. SAG 36.88 only 30% recovery were recorded (Fig.4). ΔF/Fm' of I. terricola SAG 2100, I. massjukiae SAG 2102, and Interfilum sp. SAG 2147 fully recovered after 1,000–2,500 min rehydration. In contrast, in Interfilum sp. SAG 36.88 even after 2,500 min recovery period, a maximum of only 60% of the control effective quantum yield was attained (Fig.4).
Light requirements for photosynthesis
Increasing photon fluence densities stimulated the photosynthetic oxygen production in the four Interfilum strains resulting in PI curves from which characteristic parameters for the description of the light requirements could be derived (Table2). While I. massjukiae SAG 2102, Interfilum sp. SAG 36.88, and Interfilum sp. SAG 2147 showed all low α values (photosynthetic efficiency in the light limited range) of 4.4–5.5 μmol O2 · h−1 · mg−1 Chl a (μmol photons · m−2 · s−1)−1, this value was significantly higher in I. terricola SAG 2100 (ANOVA: F3,8 = 41.8, P < 0.05; Table2). In addition, the four Interfilum isolates exhibited Ic values (light compensation point) between 6.6 and 22.4 μmol photons · m−2 · s−1 and Ik values (initial light saturation point) ranging from 17.4 to 31.9 μmol photons · m−2 · s−1 (Table2), all of which clearly point to low light requirements for photosynthesis.
Table 2.
Photosynthesis-irradiance curve parameters of the Interfilum strains studied
| Strain number | α | Ic | Ik |
|---|---|---|---|
|
I. terricola SAG 2100 |
11.75 ± 1.41A | 6.57 ± 0.82a | 17.37 ± 1.66A |
|
I. massjukiae SAG 2102 |
4.38 ± 0.57B | 22.44 ± 3.48b | 31.90 ± 5.86B |
|
Interfilum sp. SAG 36.88 |
5.48 ± 0.86B | 12.94 ± 2.29c | 29.75 ± 4.77B |
|
Interfilum sp. SAG 2147 |
4.42 ± 0.73B | 10.15 ± 1.99c | 18.92 ± 2.12A |
Data were recorded as oxygen evolution using an oxygen optode at 25°C and fitted with the photosynthesis model of Webb et al. (1974) without photoinhibition. α: positive slope at limiting photon fluence rates (μmol O2 · h−1 · mg−1 Chl a (μmol photons · m−2 · s−1)-1; Ic: light compensation point (μmol photons · m−2 · s−1); Ik: initial value of light-saturated photosynthesis (μmol photons · m−2 · s−1). Data represent mean values ± SD of three replicates. The significance of differences among the strains, as indicated by different letters (capital letters for α; small letters for Ic; cursive capital letters for Ik) was calculated by one-way ANOVA (α, F3,8 = 41.8, P < 0.05; Ic, F3,8 = 25.2, P < 0.001; Ik, F3,8 = 10.2, P < 0.05) and Tukey's post hoc test.
Although the rETR curves pointed to different maximum values for the four Interfilum strains ranging from 8 to 32 (rETRmax), no indication of photoinhibition up to the maximum photon fluence rate tested (1,432 μmol photons · m−2 · s−1) was observed (Fig.5).
Figure 5.
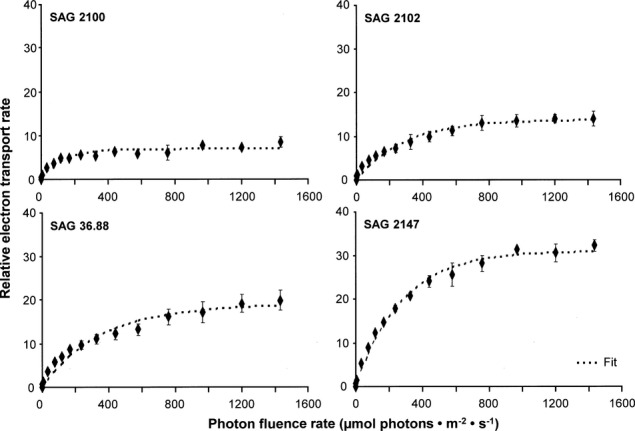
The effect of increasing photon fluence densities up to 1,432 μmol photons · m−2 · s−1 on the relative electron transport rate (rETR) in the four studied strains of Interfilum (n = 4, mean value ± SD). SAG2100: Interfilum terricola; SAG2102: Interfilum massjukiae; SAG36.88: Interfilum sp.; SAG2147: Interfilum sp. All measurements were done at 22°C ± 1°C.
Temperature requirements for photosynthesis and respiration
The effect of increasing temperatures on gross and net photosynthetic oxygen production and respiratory oxygen consumption in the four Interfilum strains clearly indicated (i) isolate-specific differences, and (ii) strong differences in sensitivity between both physiological processes (Fig.6). In all samples, respiration at 5°C and 10°C was not detectable or very low, while temperatures between >10°C and 30°C–35°C led to a continuous, and mainly linear increase in respiratory activity up to the maximum values of −60 to −150 μmol O2 · h−1 · mg−1 Chl a (I. terricola SAG 2100, F8,18 = 76.8, P < 0.01; I. massjukiae SAG 2102, F8,18 = 59.7, P < 0.01; Interfilum sp. SAG 36.88, F8,18 = 57.1, P < 0.05; Interfilum sp. SAG 2147, F8,18 = 23.3, P < 0.01; Fig.6). Further increases in temperature up to 45°C led to a moderate or strong decrease in respiration in I. terricola SAG 2100, I. massjukiae SAG 2102, and Interfilum sp. SAG 2147, while Interfilum sp. SAG 36.88 showed an unaffected respiratory rate at this high temperature (Fig.6).
Figure 6.
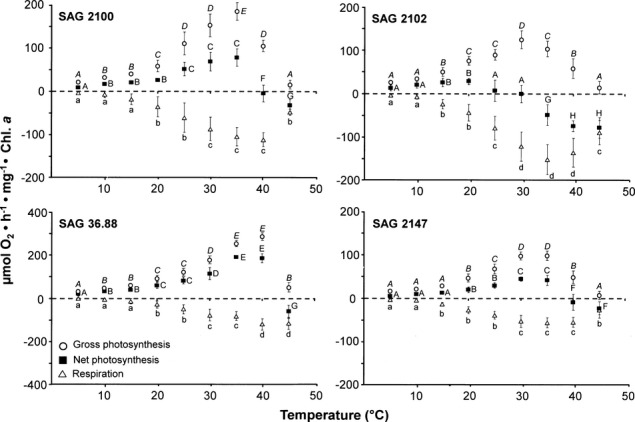
Gross and net photosynthetic oxygen development and respiratory oxygen consumption in μmol O2 · h−1 · mg−1 Chl a measured at 200 μmol photons · m−2 · s−1 as function of increasing temperatures in the four studied strains of Interfilum (n = 3, mean value ± SD). SAG2100: Interfilum terricola; SAG2102: Interfilum massjukiae; SAG36.88: Interfilum sp.; SAG2147: Interfilum sp. Note the different y-axis scale for SAG36.88. Significances among the treatments were calculated by one-way ANOVA (gross photosynthesis: I. terricola SAG 2100, F8,18 = 101.3, P < 0.01; I. massjukiae SAG 2102, F8,18 = 92.2, P < 0.01; Interfilum sp. SAG 36.88, F8,18 = 181.4, P < 0.01; Interfilum sp. SAG 2147, F8,18 = 65.3, P < 0.05; net photosynthesis: I. terricola SAG 2100, F8,18 = 88.4, P < 0.01; I. massjukiae SAG 2102, F8,18 = 83.3, P < 0.05; Interfilum sp. SAG 36.88, F8,18 = 233.7, P < 0.05; Interfilum sp. SAG 2147, F8,18 = 37.3, P < 0.01; respiration: I. terricola SAG 2100, F8,18 = 76.8, P < 0.01; I. massjukiae SAG 2102, F8,18 = 59.7, P < 0.01; Interfilum sp. SAG 36.88, F8,18 = 57.1, P < 0.05; Interfilum sp. SAG 2147, F8,18 = 23.3, P < 0.01). Different cursive capital (gross photosynthesis), capital (net photosynthesis), and small letters (respiration) represent significant differences among the temperatures as revealed by Tukey's post hoc test.
In contrast to respiration, net photosynthesis (NP) at the lowest temperatures (5°C and 10°C) was within the limit of detection and continuously and significantly increased between >10°C and 30°C–35°C in I. terricola SAG 2100, Interfilum sp. SAG 36.88, and Interfilum sp. SAG 2147, however with different isolate-specific maximum rates ranging from 50 to 180 μmol O2 · h−1 · mg−1 Chl a (I. terricola SAG 2100, F8,18 = 88.4, P < 0.01; I. massjukiae SAG 2102, F8,18 = 83.3, P < 0.05; Interfilum sp. SAG 36.88, F8,18 = 233.7, P < 0.01; Interfilum sp. SAG 2147, F8,18 = 37.3, P < 0.001; Fig.6). While strain Interfilum sp. SAG 36.88 exhibited highest net photosynthetic rate at 40°C followed by complete inhibition at 45°C, NP in I. terricola SAG 2100 and Interfilum sp. SAG 2147 was already inhibited at 40°C. In contrast to these isolates, I. massjukiae SAG 2102 showed a much higher susceptibility to temperature. Optimum NP was measured at 20°C, and it continuously decreased between 25°C and 30°C to complete inhibition (Fig.6). Since NP represents the difference of gross photosynthesis (GP) and respiration (R; NP = GP − R), GP data of the four Interfilum strains showed a similar course with increasing temperature compared to net photosynthesis, but with always positive values (Fig.6).
The gross photosynthesis:respiration (P:R) ratios in all four Interfilum strains decreased with increasing temperatures (I. terricola SAG 2100, F8,18 = 6.4, P < 0.001; I. massjukiae SAG 2102, F8,18 = 23.2, P < 0.05; Interfilum sp. SAG 36.88, F8,18 = 15.8, P < 0.05; Interfilum sp. SAG 2147, F8,18 = 11.7, P < 0.05; Fig.7). While at 5°C and 10°C, high P:R values were recorded in all isolates, they dropped in I. terricola SAG 2100 and Interfilum sp. SAG 36.88 to negative values only at 45°C (Fig.7). In SAG I. massjukiae 2102 and Interfilum sp. SAG 2147, the P.R ratios were negative between 35°C and 40°C.
Figure 7.
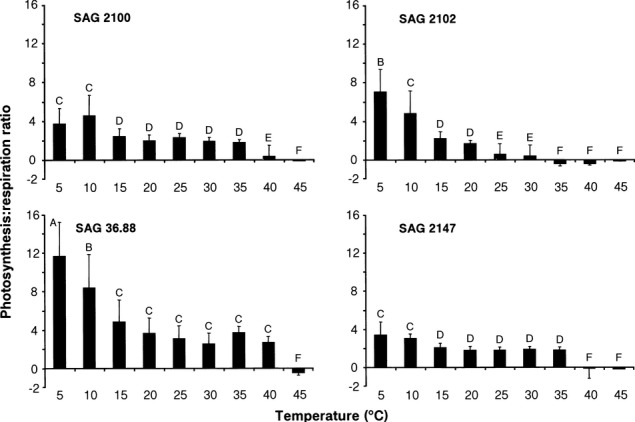
Gross photosynthesis:respiration (P:R) ratios as function of increasing temperatures in the four studied strains of Interfilum (n = 3, mean value ± SD). SAG2100: Interfilum terricola; SAG2102: Interfilum massjukiae; SAG36.88: Interfilum sp.; SAG2147: Interfilum sp. Significances among the treatments were calculated by one-way ANOVA (I. terricola SAG 2100, F8,18 = 6.4, p < 0.001; I. massjukiae SAG 2102, F8,18 = 23.2, p < 0.05; Interfilum sp. SAG 36.88, F8,18 = 15.8, p < 0.05; Interfilum sp. SAG 2147, F8,18 = 11.7, p < 0.05) Different capital letters represent significant differences among the temperatures as revealed by Tukey's post hoc test.
Discussion
Water availability is the ecological key factor for all aeroterrestrial algae. Dehydration of the four Interfilum strains under standardized conditions resulted in a strong inhibition of the effective quantum yield of PSII (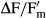 ). However, there were conspicuous interspecific differences in the response kinetics (Fig.3). I. terricola SAG 2100 showed a continuous decrease in
). However, there were conspicuous interspecific differences in the response kinetics (Fig.3). I. terricola SAG 2100 showed a continuous decrease in 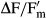 from beginning on and Interfilum sp. SAG 36.88 had decrease in
from beginning on and Interfilum sp. SAG 36.88 had decrease in 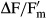 after a relatively short period of 210 min. Both isolates typically occurred as single cells. In contrast, the dehydration interval was much longer in I. massjukiae SAG 2102 and Interfilum sp. SAG 2147 before
after a relatively short period of 210 min. Both isolates typically occurred as single cells. In contrast, the dehydration interval was much longer in I. massjukiae SAG 2102 and Interfilum sp. SAG 2147 before 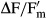 decreased, and both of these strains formed cell packets or sarcinoid colonies. These data indicate that single aeroterrestrial algal cells, which are strongly associated with other algal cells in an aggregate, colony or biofilm, are better protected against water loss and possibly other environmental stresses. The formation of such cell pockets could be related to a joint matrix of extracellular polysaccharides (EPS; mucilage) in which the cells are embedded. For instance, such mucilage surrounds the colony-forming cells of the terrestrial cyanobacterium Nostoc commune and plays an important role in increasing its tolerance of photosynthesis during dehydration (Tamaru et al. 2005). However, the strains I. massjukiae SAG 2102 and Interfilum sp. 2147 lacked mucilage as previously reported (Mikhailyuk et al. 2008). Instead the formation of aggregates was due to incorporating parts of the detaching mother cell wall in the growing daughter cell walls resulting in sarcinoid growth (Mikhailyuk et al. 2008). In contrast I. terricola SAG 2100 and Interfilum sp. SAG 36.88 had a mucilage envelope as described also by Mikhailyuk et al. (2008). The occurrence of mucilage might compensate for their inability to form distinct cell packages as a strategy to withstand water loss (Shephard 1987). All other observed morphological features in the investigated Interfilum strains corroborate earlier findings in the respective clades A1–A4 (Mikhailyuk et al. 2008, Rindi et al. 2011).
decreased, and both of these strains formed cell packets or sarcinoid colonies. These data indicate that single aeroterrestrial algal cells, which are strongly associated with other algal cells in an aggregate, colony or biofilm, are better protected against water loss and possibly other environmental stresses. The formation of such cell pockets could be related to a joint matrix of extracellular polysaccharides (EPS; mucilage) in which the cells are embedded. For instance, such mucilage surrounds the colony-forming cells of the terrestrial cyanobacterium Nostoc commune and plays an important role in increasing its tolerance of photosynthesis during dehydration (Tamaru et al. 2005). However, the strains I. massjukiae SAG 2102 and Interfilum sp. 2147 lacked mucilage as previously reported (Mikhailyuk et al. 2008). Instead the formation of aggregates was due to incorporating parts of the detaching mother cell wall in the growing daughter cell walls resulting in sarcinoid growth (Mikhailyuk et al. 2008). In contrast I. terricola SAG 2100 and Interfilum sp. SAG 36.88 had a mucilage envelope as described also by Mikhailyuk et al. (2008). The occurrence of mucilage might compensate for their inability to form distinct cell packages as a strategy to withstand water loss (Shephard 1987). All other observed morphological features in the investigated Interfilum strains corroborate earlier findings in the respective clades A1–A4 (Mikhailyuk et al. 2008, Rindi et al. 2011).
Packet-like aggregation of cells is typical for many terrestrial green algae from different phylogenetic lineages (Lopez-Bautista et al. 2008, Rindi et al. 2009). The most widespread and successful terrestrial unicellular algal taxa include the genera Apatococcus, Chlorosarcinopsis, Chlorosarcina, Chlorokybus, Desmococcus, Trebouxia, Tetracystis, and Interfilum (Hoffmann 1989, Lopez-Bautista et al. 2008, Rindi et al. 2011). The cell “self-protection” in these aggregates is considered as adaptation of algal cells to retain cellular water (Nienow 1996). Recent investigation of freshwater Coleochete species under simulated terrestrial culture conditions showed a strong change in morphology from typical radial thallus to the formation of packet-like structures (Graham et al. 2012). Therefore, the formation of a packet-like morphotype might be a general strategy of algae to thrive under terrestrial conditions.
From an ecological view, the differences in the dehydration response kinetics of the four Interfilum strains also reflected meteorological data in the respective natural habitat. I. terricola SAG 2100 and Interfilum sp. SAG 36.88 (Table1) were isolated from soils under oceanic, humid influence, which may explain their higher susceptibility against water loss. Both collecting locations in Belgium and New Zealand are characterized by a marine coastal climate that typically is mild with no dry season, warm summers, and moderate seasonality (Köppen-Geiger classification: Cfb; Peel et al. 2007). In contrast, I. massjukiae SAG 2102 and Interfilum sp. SAG 2147 (Table1) were collected from drier habitats with continental warm summers (Köppen-Geiger classification: Dfb; Peel et al. 2007). The Interfilum strains maintained in culture under identical abiotic conditions for long periods still exhibited conspicuous differences in morphology, aggregation, mucilage formation, and ecophysiological response patterns, which indicates that these aeroterrestrial algae did not lose their genetic traits.
Recovery kinetics of 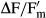 after rehydration of the dried Interfilum samples also showed conspicuous interspecific differences. While I. terricola SAG 2100, I. massjukiae SAG 2102, and Interfilum sp. SAG 2147 fully recovered upon rehydration, although after different time intervals, Interfilum sp. SAG 36.88 only partly recovered (60% of control) which points to some damage in the photosynthetic apparatus. In the microscopic observations, this strain appeared to be the most fragile with a higher surface to volume ratio when compared to the elliptic cells of the other strains. In another study, the optimum quantum yield as a function of changing water availability was followed over several months on an aeroterrestrial green algal biofilm growing on anthropogenic hard substrata (Häubner et al. 2006). This biofilm, which was dominated by unicellular taxa, was controlled by dew; with increasing insolation after sunrise, algal cells continuously lost water resulting in inhibition of photosynthesis. After artificial moistening, however, quick recovery of photosynthesis was observed (Häubner et al. 2006), which was similar to the recovery kinetics in Interfilum strains analyzed in the present study. Such high dehydration tolerance in conjunction with high recovery rates seems to be a typical feature of many aeroterrestrial green algae from dune, alpine, and dryland soil crust communities (De Winder et al. 1990, Gray et al. 2007, Karsten et al. 2010, Karsten and Holzinger 2012).
after rehydration of the dried Interfilum samples also showed conspicuous interspecific differences. While I. terricola SAG 2100, I. massjukiae SAG 2102, and Interfilum sp. SAG 2147 fully recovered upon rehydration, although after different time intervals, Interfilum sp. SAG 36.88 only partly recovered (60% of control) which points to some damage in the photosynthetic apparatus. In the microscopic observations, this strain appeared to be the most fragile with a higher surface to volume ratio when compared to the elliptic cells of the other strains. In another study, the optimum quantum yield as a function of changing water availability was followed over several months on an aeroterrestrial green algal biofilm growing on anthropogenic hard substrata (Häubner et al. 2006). This biofilm, which was dominated by unicellular taxa, was controlled by dew; with increasing insolation after sunrise, algal cells continuously lost water resulting in inhibition of photosynthesis. After artificial moistening, however, quick recovery of photosynthesis was observed (Häubner et al. 2006), which was similar to the recovery kinetics in Interfilum strains analyzed in the present study. Such high dehydration tolerance in conjunction with high recovery rates seems to be a typical feature of many aeroterrestrial green algae from dune, alpine, and dryland soil crust communities (De Winder et al. 1990, Gray et al. 2007, Karsten et al. 2010, Karsten and Holzinger 2012).
The mechanisms involved in desiccation tolerance of green algae such as Interfilum or Klebsormidium are still poorly understood. In general, desiccation-tolerant plants can be divided into two groups according to Oliver and Bewley (1997): those classified as “modified desiccation-tolerant plants” (some “higher plants”, i.e., resurrection plants) and the “fully desiccation-tolerant plants” (some bryophytes, small pteridophytes, and aeroterrestrial algae). Tolerant “higher plants” only survive if dehydration is moderate and very slow, so that full recovery is guaranteed. The main strategy of these plants is to induce protective mechanisms during desiccation, rather than repair upon rehydration (Alpert and Oliver 2002). In contrast, aeroterrestrial algae and cyanobacteria can survive very rapid drying events for a long time and quickly recover after rehydration (Potts 2001). However, molecular and biochemical mechanisms involved in desiccation tolerance of “higher” and “lower plants” have not been investigated so far in Interfilum and Klebsormidium.
Aeroterrestrial green algae are confronted with problems in carbon dioxide supply for photosynthesis as a function of their cellular water status, since the diffusion of this gas is almost 9,000-fold higher in air as in water (Green et al. 2008). Consequently, the precise water status of Interfilum should ideally always be known during experimentation, but this is methodologically challenging, and hence could not be considered in the present study. In Klebsormidium flaccidum (De Winder et al. 1990) and various lichens (Green et al. 2008), it could be shown that strong loss of water resulted in depression of photosynthesis if species-specific cell water concentrations go below this value, which is in principle confirmed by the Interfilum data. In addition, too much water, e.g., after strong precipitation, had a negative effect on photosynthesis in lichens. The inhibited photosynthesis under these conditions was related to the establishment of an external water film, which acted as a barrier by reducing carbon dioxide diffusion from the air into the algal cells (Green et al. 2008).
Measurements of PI curves enable the light requirements of algae for photosynthesis to be characterized, particularly in the context of fluctuating radiation conditions (Henley 1993). High α together with low Ic and Ik values, as measured in the four Interfilum strains, are typical for algae living under shaded conditions. Although microclimatic information on the natural habitats of the Interfilum species are missing, we know from our field observations on Klebsormidium and from Dr. T. Mikhailyuk (pers comm), that it appears to prefer low radiation conditions such as under the canopy of oak trees (I. terricola SAG 2100; Table1). However, in the present study PAM measurements under enhanced photon fluence rates clearly documented that all Interfilum isolates were not photoinhibited. This is a rather unusual photophysiological response pattern, as in most algae studied so far low radiation adaptation of photosynthesis is typically reflected in photoinhibition under high light conditions (e.g., Bischof et al. 1998). From the few data available on Klebsormidium, it seems that such a photophysiological plasticity is a common trait for soil crust green algae to better cope with strong fluctuating changes in the natural light climate (Kaplan et al. 2012, Karsten and Holzinger 2012). In “higher plants” and various algal groups, a set of mechanisms have been described which limit the extent of photodamage and/or which efficiently repair photodamage (Raven 2011). These mechanisms include partial avoidance of photodamage by restricting the number of photons incident on the photosynthetic apparatus. For example, phototactic movement of plastids within cells to minimize the absorption of incident PAR. Other avoiding mechanisms typically aim to dissipate excitation of photosynthetic pigments, e.g., by nonphotochemical quenching (e.g., xanthophyll cycle) or photochemical quenching (e.g., alternative electron transport pathways) (Raven 2011). The reason for the lack of photoinhibition in all Interfilum isolates might be explained by one of these mechanisms, but needs to be further investigated.
The effect of increasing temperatures on respiratory oxygen consumption and photosynthetic oxygen production in the four Interfilum isolates showed intraspecific, as well as strong differences in the temperature requirements of both physiological processes. While three of four Interfilum strains exhibited optimum photosynthesis between 30°C and 40°C, one isolate was already strongly inhibited under these temperatures, and at 45°C none of the isolates showed oxygen evolution. In contrast, respiration of all strains was not detectable at low temperatures, and was highest between 35°C and 45°C. The emerging picture is that photosynthesis under lower temperatures is generally more efficiently functioning than respiration, while the opposite is true for higher temperatures, where respiration typically exhibits enhanced activity rates compared to photosynthesis. This is also in agreement with the P:R ratios (Fig.7), which confirm a high net carbon gain and hence biomass formation mainly under temperate conditions, thereby reflecting the natural habitats of the four Interfilum strains. Consequently, the physiological requirements point to optimum in situ photosynthesis activity periods of Interfilum during cool and moist spring and autumn in temperate regions compared to the often rather dry and warm summer months (http://www.worldclimate.com). This disproportionate effect of temperature on two of the key physiological processes in aeroterrestrial green algae has also been documented in the closely related Klebsormidium crenulatum and Klebsormidium dissectum (Karsten et al. 2010, Karsten and Holzinger 2012), as well as in Prasiola crispa from Antarctica (Davey 1989). The latter species is a macroalgal member of the Trebouxiophyceae (Chlorophyta), and hence from an evolutionary standpoint far distant from Interfilum and Klebsormidium. Nevertheless, similar physiological response patterns with rising temperatures strongly support the assumption that aeroterrestrial green algae in general, and independent of their phylogenetic position, exhibit this trait. The conspicuously different temperature requirements for photosynthesis and respiration can be explained by the fact that the first process is more dependent on light-related processes (light absorption, energy transfer etc.) than on temperature, while the second one is completely controlled by temperature (Atkin and Tjoelker 2003). Algal respiration consists of a set of catabolic reactions, localized in different cellular compartments and controlled by a whole array of specific enzymes, of which many exhibit different temperature optima. One partly inhibited respiratory enzyme under cool conditions would be sufficient to act as a bottleneck affecting the whole process (Atkin and Tjoelker 2003).
In conclusion, the aeroterrestrial Interfilum isolates investigated for the first time in the present study, exhibited a high dehydration tolerance, low light requirements for photosynthesis in combination with a lack of photoinhibition, and a relatively high temperature tolerance compared to the monthly maximum air temperature of the respective habitats (Table1), which concur with the regular occurrence in temperate soils or temperate biological soil crust communities. Although water availability seems to be the ecological key factor for aeroterrestrial algae, there exists of course under natural conditions an interrelationship of all environmental factors, which affect and control the optimum water content in terrestrial habitats. However, further information on inter- and infraspecific differentiation in Interfilum is urgently needed, to better understand physiological plasticity and adaptive strategies of this genus along with state-of-the-art approaches in genomics and transcriptomics.
The present study was undertaken during the first author's sabbatical at the University of Innsbruck. Financial support by the Deutsche Forschungsgemeinschaft (DFG; KA899/16-1/2/3/4) is gratefully acknowledged by U.K. The study has been supported by FWF grant P24242-B16 to A.H.
Glossary
- EPS
extracellular polymeric substances
- PAM
pulse amplitude modulation fluorometry
- PAR
photosynthetic active radiation
- PFD
photon flux density
- PSII
photosystem II
- RAH
relative air humidity
- rETR
relative electron transport rate
References
- Alpert P. Oliver MJ. Drying without dying. In: Pritchard HW, editor; Black M, editor. Desiccation and Survival in Plants: Drying Without Dying. Wallingford, Oxon: CABI Publishing; 2002. pp. 3–43. [Google Scholar]
- Atkin OK. Tjoelker MG. Thermal acclimation and the dynamic response of plant respiration to temperature. Trends Plant Sci. 2003;8:343–51. doi: 10.1016/S1360-1385(03)00136-5. [DOI] [PubMed] [Google Scholar]
- Belnap J. Lange OL. Biological Soil Crusts: Structure, Function, and Management. Berlin, Germany: Springer; 2001. p. 503. [Google Scholar]
- Bischof K, Hanelt D, Tüg H, Karsten U, Brouwer PEM. Wiencke C. Acclimation of brown algal photosynthesis to ultraviolet radiation in Arctic coastal waters (Spitsbergen, Norway) Polar Biol. 1998;20:388–95. [Google Scholar]
- Brown RM, Larson DA. Bold HC. Airborne algae: their abundance and heterogeneity. Science. 1964;143:583–5. doi: 10.1126/science.143.3606.583. [DOI] [PubMed] [Google Scholar]
- Castillo-Monroy AP, Maestre FT, Delgado-Baquerizo M. Gallardo A. Biological soil crust modulate nitrogen availability in semi-arid ecosystem: insights from a Mediterranean grassland. Plant Soil. 2010;333:21–34. [Google Scholar]
- Davey MC. The effects of freezing and desiccation on photosynthesis and survival of terrestrial Antarctic algae and cyanobacteria. Polar Biol. 1989;10:29–36. [Google Scholar]
- De Winder B, Matthijs HCP. Mur LR. The effect of dehydration and ion stress on carbon dioxide fixation in drought-tolerant phototrophic micro-organisms. FEMS Microbiol. Ecol. 1990;74:33–8. [Google Scholar]
- Ehrenhaus C. Vigna MS. Interfilum paradoxum var. regulare var. nov. (Chlorococcales, Chlorophyta) primer registro del género para América. Darwiniana. 2008;46:46–50. [Google Scholar]
- Elbert W, Weber B, Burrows S, Steinkamp J, Büdel B, Andreae MO. Pöschl U. Contribution of crytogamic covers to the global cycles of carbon and nitrogen. Nature Geosci. 2012;5:459–62. [Google Scholar]
- Ettl H. Gärtner G. Syllabus der Boden-, Luft- und Flechtenalgen. Stuttgart, Germany: Gustav Fischer; 1995. p. 721. [Google Scholar]
- Evans RD. Johansen JR. Microbiotic crusts and ecosystem processes. Crit. Rev. Plant Sci. 1999;18:83–225. [Google Scholar]
- Flechtner VR. North American desert microbiotic soil crust communities: diversity despite challenge. In: Seckbach J, editor. Algae and Cyanobacteria in Extreme Environments. Berlin, Germany: Springer; 2007. pp. 537–51. [Google Scholar]
- Friedl T. Rybalka N. Systematics of the green algae: a brief introduction to the current status. In: Weigend M, editor; Lüttge U, Beyschlag W, Cushman J, editors. Progress in Botany. Vol. 73. Berlin, Germany: Springer; 2012. pp. 259–80. [Google Scholar]
- Genty B, Briantais JM. Baker NR. The relationship between the quantum yield of photosynthetic electron-transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta. 1989;990:87–92. [Google Scholar]
- Graham LE, Arancibia-Avila P, Taylor WA, Strother PK. Cook ME. Aeroterrestrial Coleochaete (Streptophyta, Coleochaetales) models early plant adaptation to land. Am. J. Bot. 2012;99:130–44. doi: 10.3732/ajb.1100245. [DOI] [PubMed] [Google Scholar]
- Gray DW, Lewis LA. Cardon ZG. Photosynthetic recovery following desiccation of desert green algae (Chlorophyta) and their aquatic relatives. Plant, Cell Environ. 2007;30:1240–55. doi: 10.1111/j.1365-3040.2007.01704.x. [DOI] [PubMed] [Google Scholar]
- Green TGA, Nash TH., III Lange OL. Physiological ecology of carbon dioxide exchange. In: Nash TH III, editor; Lichen Biology. Cambridge, UK: Cambridge University Press; 2008. pp. 152–81. [Google Scholar]
- Guillard RRL. Sieracki MS. Counting cells in cultures with the light microscope. In: Andersen RA, editor; Algal Culturing Techniques. Waltham, MA: Academic Press; 2005. pp. 239–52. [Google Scholar]
- Häubner N, Schumann R. Karsten U. Aeroterrestrial algae growing on facades – response to temperature and water stress. Microb. Ecol. 2006;51:285–93. doi: 10.1007/s00248-006-9016-1. [DOI] [PubMed] [Google Scholar]
- Henley WJ. Measurement and interpretation of photosynthetic light-response curves in algae in the context of photoinhibition and diel changes. J. Phycol. 1993;29:729–39. [Google Scholar]
- Hoffmann L. Algae of terrestrial habitats. Bot. Rev. 1989;55:77–105. [Google Scholar]
- Holzinger A. Karsten U. Desiccation stress and tolerance in green algae: consequences for ultrastructure, physiological and molecular mechanisms. Frontiers Plant Sci. 2013;4:article 327. doi: 10.3389/fpls.2013.00327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzinger A, Lütz C. Karsten U. Desiccation stress causes structural and ultra-structural alterations in the aeroterrestrial green alga Klebsormidium crenulatum (Klebsormidiophyceae, Streptophyta) isolated from an alpine soil crust. J. Phycol. 2011;47:591–602. doi: 10.1111/j.1529-8817.2011.00980.x. [DOI] [PubMed] [Google Scholar]
- Kaplan F, Lewis LA, Wastian J. Holzinger A. Plasmolysis effects and osmotic potential of two phylogenetically distinct alpine strains of Klebsormidium (Streptophyta) Protoplasma. 2012;249:789–804. doi: 10.1007/s00709-011-0324-z. [DOI] [PubMed] [Google Scholar]
- Karsten U. Holzinger A. Light, temperature and desiccation effects on photosynthetic activity, and drought-induced ultrastructural changes in the green alga Klebsormidium disectum (Streptophyta) from a high alpine soil crust. Microb. Ecol. 2012;63:51–63. doi: 10.1007/s00248-011-9924-6. [DOI] [PubMed] [Google Scholar]
- Karsten U, Lütz C. Holzinger A. Ecophysiological performance of the aeroterrestrial green alga Klebsormidium crenulatum (Klebsormidiophyceae, Streptophyta) isolated from an alpine soil crust with an emphasis on desiccation stress. J. Phycol. 2010;46:1187–97. doi: 10.1111/j.1529-8817.2011.00980.x. [DOI] [PubMed] [Google Scholar]
- Karsten U. Rindi F. Ecophysiological performance of an urban strain of the aeroterrestrial green alga Klebsormidium sp. (Klebsormidiales, Klebsormidiophyceae) Eur. J. Phycol. 2010;45:426–35. [Google Scholar]
- Karsten U, Schumann R. Mostaert A. Aeroterrestrial algae growing on man-made surfaces – what are the secrets of their ecological success? In: Seckbach J, editor; Algae and Cyanobacteria in Extreme Environments. Berlin, Germany: Springer; 2007. pp. 583–97. [Google Scholar]
- Kromkamp JC. Forster RM. The use of variable fluorescence measurements in aquatic ecosystems: differences between multiple and single turnover measuring protocols and suggested terminology. Eur. J. Phycol. 2003;38:103–12. [Google Scholar]
- Leliaert F, Smith DR, Moreau H, Herron MD, Verbruggen H, Delwiche CF. De Clerck O. Phylogeny and molecular evolution of the green algae. Crit. Rev. Plant Sci. 2012;31:1–46. [Google Scholar]
- Lewis LA. Chlorophyta on land: independent lineages of green eukaryotes from arid lands. In: Seckbach J, editor. Algae and Cyanobacteria in Extreme Environments. Berlin, Germany: Springer; 2007. pp. 571–82. [Google Scholar]
- Lopez-Bautista JM, Rindi F. Casamatta D. The systematics of subaerial algae. In: Seckbach J, editor; Algae and Cyanobacteria in Extreme Environments. Berlin, Germany: Springer; 2008. pp. 599–617. [Google Scholar]
- Mikhailyuk TI, Sluiman HJ, Massalski A, Mudimu O, Demchenko EM, Kondratyuk SY. Friedl T. New streptophyte green algae from terrestrial habitats and an assessment of the genus Interfilum (Klebsormidiophyceae, Streptophyta) J. Phycol. 2008;44:1586–603. doi: 10.1111/j.1529-8817.2008.00606.x. [DOI] [PubMed] [Google Scholar]
- Nienow JA. Ecology of subaerial algae. Nowa Hed. Beiheft. 1996;112:537–52. [Google Scholar]
- Novis PM. Visnovsky G. Novel alpine algae for New Zealand: Klebsormidiale. New Zeal. J. Bot. 2011;49:339–49. [Google Scholar]
- Oliver MJ. Bewley JD. Desiccation tolerance of plant tissues: a mechanistic overview. Horticult. Rev. 1997;18:171–214. [Google Scholar]
- Peel MC, Finlayson BL. McMahon TA. Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 2007;11:1633–44. [Google Scholar]
- Pointing SB. Belnap J. Microbial colonization and controls in dryland systems. Nat. Rev. Microbiol. 2012;10:551–62. doi: 10.1038/nrmicro2831. [DOI] [PubMed] [Google Scholar]
- Porra RJ, Thompson WA. Kriedmann PE. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta. 1989;975:384–94. [Google Scholar]
- Potts M. Desiccation tolerance: a simple process? Trends Microbiol. 2001;9:553–9. doi: 10.1016/s0966-842x(01)02231-4. [DOI] [PubMed] [Google Scholar]
- Raven JA. The cost of photoinhibition. Physiol. Plant. 2011;142:87–104. doi: 10.1111/j.1399-3054.2011.01465.x. [DOI] [PubMed] [Google Scholar]
- Remias D, Albert A. Lütz C. Effects of simulated, but realistic, elevated UV irradiation on photosynthesis and pigment composition of the alpine snow alga Chlamydomonas nivalis and the Arctic soil alga Tetracystis sp. (Chlorophyceae) Photosynthetica. 2010;48:269–77. [Google Scholar]
- Reynolds R, Belnap J, Reheis M, Lamothe P. Luiszer F. Aeolian dust in Colorado Plateau soils: nutrient inputs and recent change in source. Proc. Natl. Acad. Sci. USA. 2001;98:7123–7. doi: 10.1073/pnas.121094298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rindi F. Diversity, distribution and ecology of green algae and cyanobacteria in urban habitats. In: Seckbach J, editor. Algae and Cyanobacteria in Extreme Environments. Berlin, Germany: Springer; 2007. pp. 621–38. [Google Scholar]
- Rindi F, Allali HA, Lam DW, Lopez-Bautista JM. An overview of the biodiversity and biogeography of terrestrial green algae. In: Rescingo V, Maletta S, editors. Biodiversity Hotspots. Hauppauge, NY: Nova Science Publishers; 2009. pp. 105–22. [Google Scholar]
- Rindi F, Mikhailyuk TI, Sluiman HJ, Friedl T. Lopez-Bautista JM. Phylogenetic relationships in Interfilum and Klebsormidium (Klebsormidiophyceae, Streptophyta) Mol. Phylogenet. Evol. 2011;58:218–31. doi: 10.1016/j.ympev.2010.11.030. [DOI] [PubMed] [Google Scholar]
- Schreiber U. Bilger W. Progress in chlorophyll fluorescence research: major developments during the past years in retrospect. Prog. Bot. 1993;54:151–73. [Google Scholar]
- Sharma N, Rai A, Singh S. Brown R. Airborne algae: their present status and relevance. J. Phycol. 2007;43:615–27. [Google Scholar]
- Shephard KL. Evaporation of water from the mucilage of a gelatinous algal community. Br. Phycol. J. 1987;22:181–5. [Google Scholar]
- Sluiman HJ, Guihal C. Mudimu O. Assessing phylogenetic affinities and species delimitations in Klebsormidiales (Streptophyta): nuclear-encoded rDNA phylogeny and ITS secondary structure models in Klebsormidium Hormidiella and Entransia. J. Phycol. 2008;44:183–95. doi: 10.1111/j.1529-8817.2007.00442.x. [DOI] [PubMed] [Google Scholar]
- Starr RC. Zeikus JA. UTEX — the culture collection of algae at the University of Texas at Austin 1993 list of cultures. J. Phycol. 1993;29:1–106. [Google Scholar]
- Tamaru Y, Takani Y, Yoshida T. Sakamoto T. Crucial role of extracellular polysaccharides in desiccation and freezing tolerance in the terrestrial cyanobacterium Nostoc commune. Appl. Environm. Microbiol. 2005;71:7327–33. doi: 10.1128/AEM.71.11.7327-7333.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb WL, Newton M. Starr D. Carbon dioxide exchange of Alnus rubra: a mathematical model. Oecologia. 1974;17:281–91. doi: 10.1007/BF00345747. [DOI] [PubMed] [Google Scholar]
- Worland MR. Lukešová A. The effect of feeding on specific soil algae on the cold-hardiness of two Antarctic micro-arthropods (Alaskozetes antarcticus and Cryptopygus antarcticus. Polar Biol. 2000;23:766–74. [Google Scholar]
- Yancey PH. Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses. J. Exp. Biol. 2005;208:2819–30. doi: 10.1242/jeb.01730. [DOI] [PubMed] [Google Scholar]


