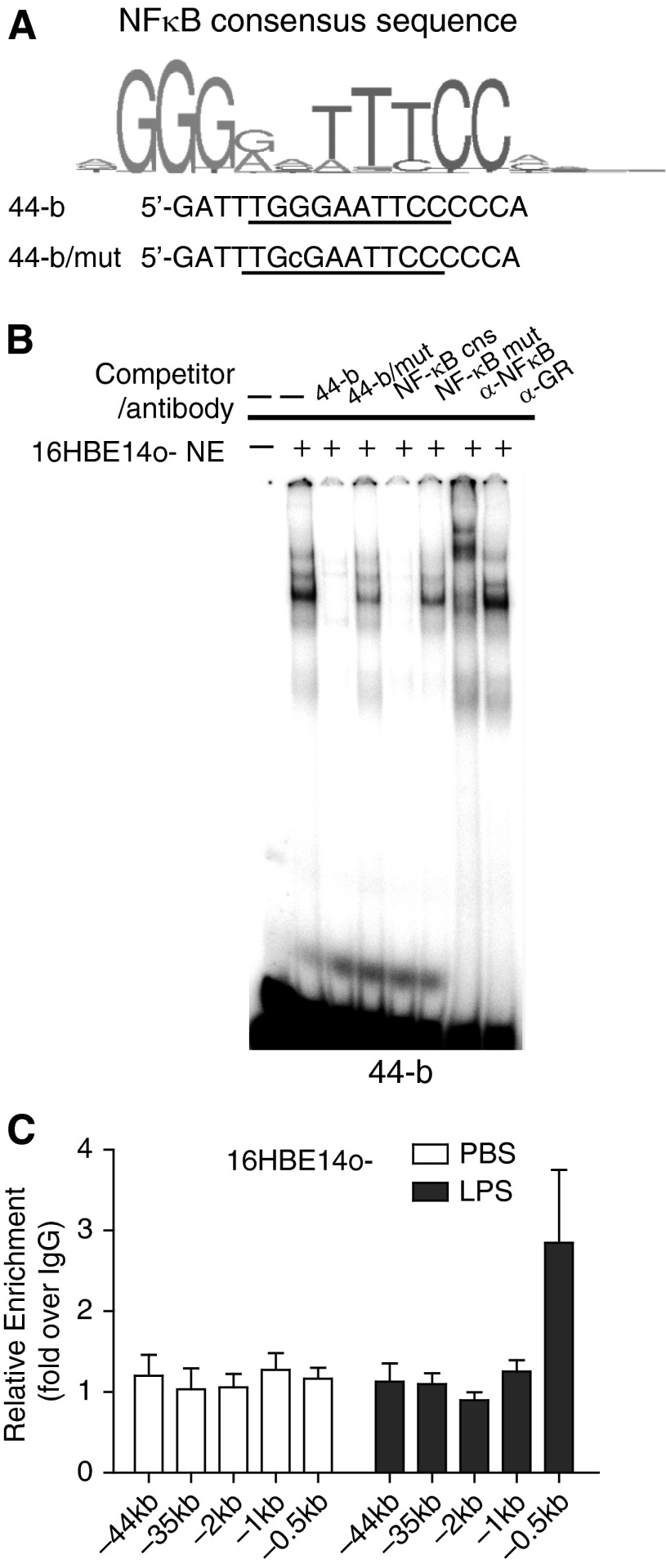
Figure 2.
NF-κB (p65) binds to a NF-κB motif in DHS-44(279) in vitro but not in vivo. (A) Identification of a predicted NF-κB binding motif in fragment 44-b and generation of a mutant version. (B) EMSAs show binding of NF-κB to 44-b in vitro. The DNA–protein complex generated when 16HBE14o- nuclear extract was incubated with a 32P-labeled 44-b probe was competed by 50× excess of unlabeled 44-b probe and an NF-κB consensus probe but not by mutant versions (Table E3). Incubation of the complex with an antibody specific for NF-κB (p65) generated a supershift (GR antibody = isotype control). (C) LPS treatment of 16HBE14o- cells increases NF-κB occupancy at the binding site in the CFTR promoter (−0.5 kb) but not at DHS-44kb. ChIP was performed with an antibody specific for NF-κB (p65). Black bars: 200 ng/ml LPS in PBS. White bars: PBS. Data normalized to IgG; error bars represent SEM of at least two ChIP experiments.