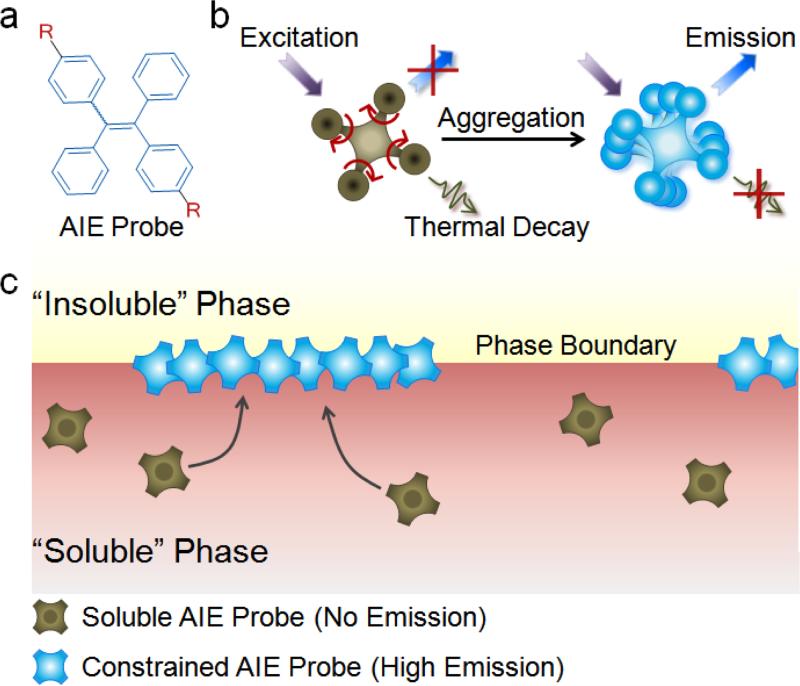
Figure 1.
a) Chemical structure of tetraphenylethene (TPE)-based AIE probe. R is a side group of an AIEgen that conveys selective solubility of the probe in one phase of an interfacial system. b) Emission enhancement by the restriction of intramolecular rotation (RIR) mechanism in AIE-active probes. When fully dissolved, the TPE phenyl rings rotate freely, dissipating the energy upon excitation in the form of heat and thus quenching fluorescence. Phase transition and probe aggregation, in turn, activate the RIR process of the phenyl rings, promoting energy dissipation by emission and dramatically increasing fluorescence intensity of AIEgens. c) Schematic of AIE-based imaging technology for real-time monitoring of the interfacial processes. In the “soluble ” phase, AIE-active probes remain non-emissive under external excitation. At the phase boundary, in contrast, probes meet the “insoluble” phase and exhibit high fluorescence because of the RIR process.