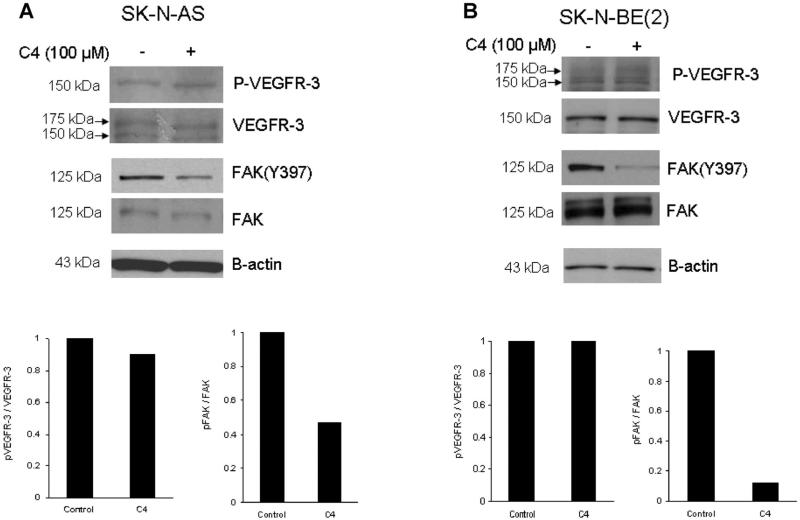
Figure 3.
C4 caused dephosphorylation of focal adhesion kinase. Immunoblotting was performed for VEGFR-3, FAK and phosphorylation of both and densitometry was used to further illustrate changes. (A) SKN-AS cells were treated with C4 at 100 μM for 24 h and were evaluated for phosphorylation of FAK and VEGFR-3. Treatment of these cells resulted in no change in the phosphorylation of Y1063/1068 VEGFR-3 (top blot and bottom left graph), but did decrease the phosphorylation of Y397 FAK (top blot and bottom right graph). (B) The SK-N-BE(2) cell line was treated with C4 at 100 μM for 24 h and phosphorylation of VEGFR-3 and FAK was examined. As seen with the SK-N-AS cells, there was no effect upon phosphorylation of VEGFR-3 (top blot and bottom left graph), but C4 treatment resulted in a marked decrease in Y397 FAK phosphorylation (top blot and bottom right graph).