Abstract
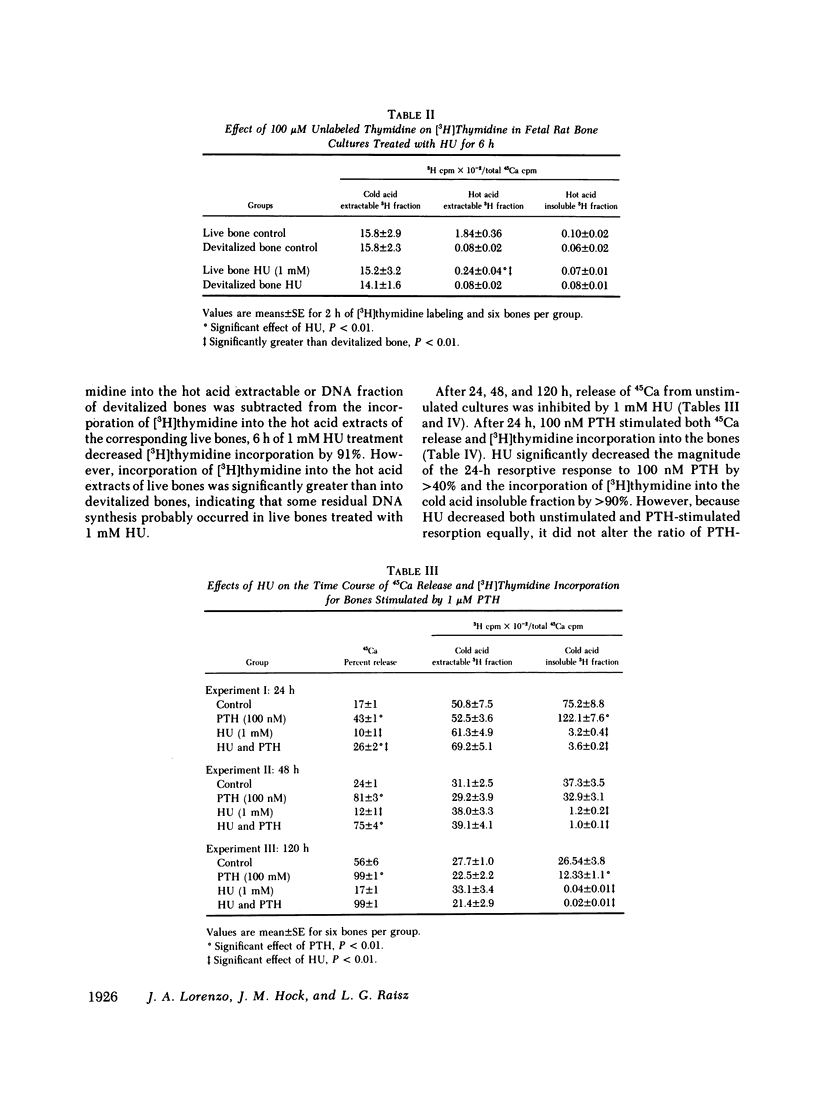
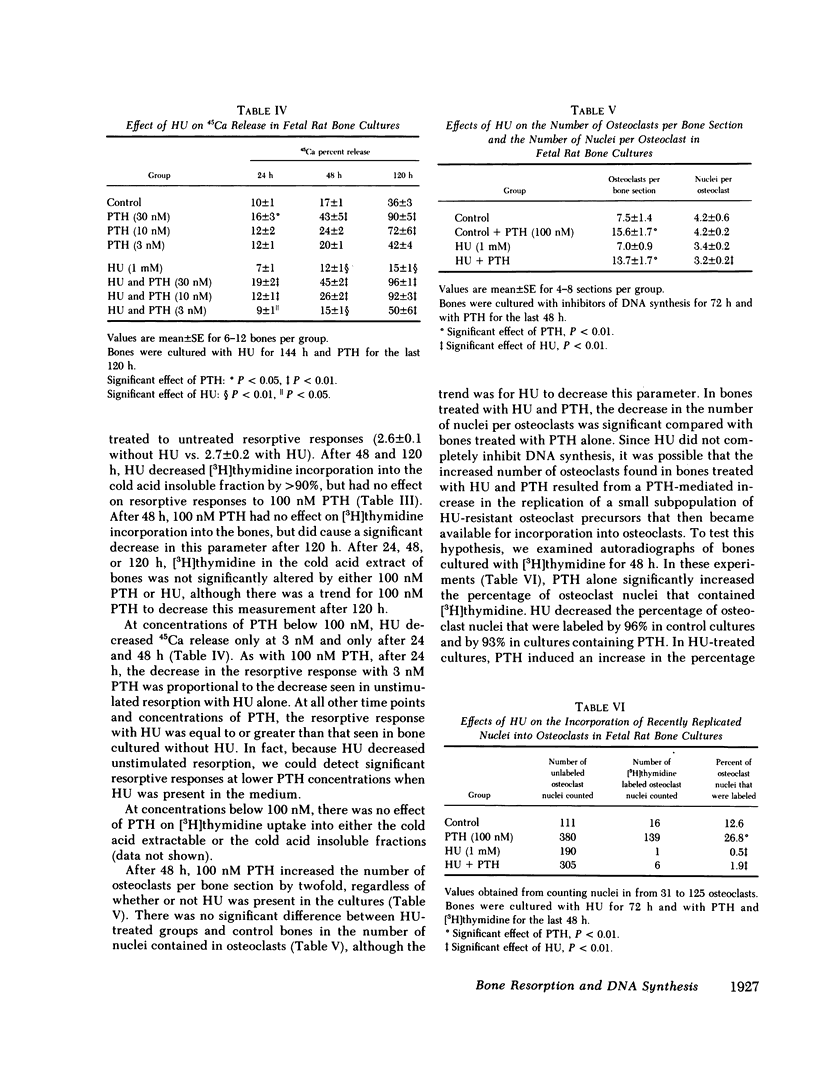
Osteoclasts, the principal cells mediating bone resorption, are believed to increase their size, number, and resorbing activity in response to parathyroid hormone (PTH) through mechanisms dependent upon the fusion of specific mononuclear precursor cells into either new or existing multinucleated osteoclasts. To address the question of whether these actions of PTH are dependent on the replication of osteoclast precursor cells, we examined the ability of an inhibitor of DNA synthesis, hydroxyurea (HU), to alter bone resorption, osteoclast formation, and DNA synthesis in cultured fetal rat bones treated with PTH. We found that HU significantly reduced [3H]thymidine incorporation into the bones and labeling of osteoclast nuclei by greater than 90%, but did not prevent PTH from stimulating bone resorption, measured as the release of 45Ca, or from increasing the number of osteoclasts in the bones. In bones cultured without PTH, HU decreased the rate of bone resorption, but not the number of osteoclasts per bone. We conclude that in fetal rat bone cultures, PTH can increase osteoclast number and stimulate bone resorption by affecting existing osteoclasts and osteoclast precursors, and that replication of osteoclast precursor cells is not necessary for PTH to stimulate a resorptive response. In unstimulated cultures it appears that HU inhibits bone resorption by affecting mechanisms that are independent of changes in osteoclast number and that may be influenced by cell replication or other unknown factors.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akerblom L., Ehrenberg A., Gräslund A., Lankinen H., Reichard P., Thelander L. Overproduction of the free radical of ribonucleotide reductase in hydroxyurea-resistant mouse fibroblast 3T6 cells. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2159–2163. doi: 10.1073/pnas.78.4.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingham P. J., Brazell I. A., Owen M. The effect of parathyroid extract on cellular activity and plasma calcium levels in vivo. J Endocrinol. 1969 Nov;45(3):387–400. doi: 10.1677/joe.0.0450387. [DOI] [PubMed] [Google Scholar]
- Canalis E. M., Dietrich J. W., Maina D. M., Raisz L. G. Hormonal control of bone collagen synthesis in vitro. Effects of insulin and glucagon. Endocrinology. 1977 Mar;100(3):668–674. doi: 10.1210/endo-100-3-668. [DOI] [PubMed] [Google Scholar]
- Canalis E., Raisz L. G. Effect of epidermal growth factor on bone formation in vitro. Endocrinology. 1979 Apr;104(4):862–869. doi: 10.1210/endo-104-4-862. [DOI] [PubMed] [Google Scholar]
- DeBartolo T. F., Pegg L. E., Shasserre C., Hahn T. J. Comparison of parathyroid hormone and calcium ionophore A23187 effects on bone resorption and nucleic acid synthesis in cultured fetal rat bone. Calcif Tissue Int. 1982 Sep;34(5):495–500. doi: 10.1007/BF02411291. [DOI] [PubMed] [Google Scholar]
- Feldman R. S., Krieger N. S., Tashjian A. H., Jr Effects of parathyroid hormone and calcitonin on osteoclast formation in vitro. Endocrinology. 1980 Oct;107(4):1137–1143. doi: 10.1210/endo-107-4-1137. [DOI] [PubMed] [Google Scholar]
- Göthlin G., Ericsson J. L. The osteoclast: review of ultrastructure, origin, and structure-function relationship. Clin Orthop Relat Res. 1976 Oct;(120):201–231. [PubMed] [Google Scholar]
- Holtrop M. E., Raisz L. G., Simmons H. A. The effects of parathyroid hormone, colchicine, and calcitonin on the ultrastructure and the activity of osteoclasts in organ culture. J Cell Biol. 1974 Feb;60(2):346–355. doi: 10.1083/jcb.60.2.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jotereau F. V., Le Douarin N. M. The development relationship between osteocytes and osteoclasts: a study using the quail-chick nuclear marker in endochondral ossification. Dev Biol. 1978 Apr;63(2):253–265. doi: 10.1016/0012-1606(78)90132-x. [DOI] [PubMed] [Google Scholar]
- Kahn A. J., Simmons D. J. Investigation of cell lineage in bone using a chimaera of chick and quial embryonic tissue. Nature. 1975 Nov 27;258(5533):325–327. doi: 10.1038/258325a0. [DOI] [PubMed] [Google Scholar]
- Krieger N. S., Feldman R. S., Tashjian A. H., Jr Parathyroid hormone and calcitonin interactions in bone: irradiation-induced inhibition of escape in vitro. Calcif Tissue Int. 1982 Mar;34(2):197–203. doi: 10.1007/BF02411233. [DOI] [PubMed] [Google Scholar]
- Liskova-Kiar M. Mode of action of cortisol on bone resorption in fetal rat fibulae cultured in vitro. Am J Anat. 1979 Sep;156(1):63–75. doi: 10.1002/aja.1001560106. [DOI] [PubMed] [Google Scholar]
- Lorenzo J. A., Raisz L. G. Divalent cation ionophores stimulate resorption and inhibit DNA synthesis in cultured fetal rat bone. Science. 1981 Jun 5;212(4499):1157–1159. doi: 10.1126/science.6785885. [DOI] [PubMed] [Google Scholar]
- Nadal-Ginard B. Commitment, fusion and biochemical differentiation of a myogenic cell line in the absence of DNA synthesis. Cell. 1978 Nov;15(3):855–864. doi: 10.1016/0092-8674(78)90270-2. [DOI] [PubMed] [Google Scholar]
- Owen M. The origin of bone cells. Int Rev Cytol. 1970;28:213–238. doi: 10.1016/s0074-7696(08)62544-9. [DOI] [PubMed] [Google Scholar]
- RAISZ L. G. BONE RESORPTION IN TISSUE CULTURE. FACTORS INFLUENCING THE RESPONSE TO PARATHYROID HORMONE. J Clin Invest. 1965 Jan;44:103–116. doi: 10.1172/JCI105117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe D. J., Hausmann E. Quantitative analyses of osteoclast changes in resorbing bone organ cultures. Calcif Tissue Res. 1977 Oct 20;23(3):283–289. doi: 10.1007/BF02012798. [DOI] [PubMed] [Google Scholar]
- Shupnik M. A., Ip N. Y., Tashjian A. H., Jr Characterization and regulation of receptors for epidermal growth factor in mouse calvaria. Endocrinology. 1980 Dec;107(6):1738–1746. doi: 10.1210/endo-107-6-1738. [DOI] [PubMed] [Google Scholar]
- Talmage R. V. A study of the effect of parathyroid hormone on bone remodeling and on calcium homeostasis. Clin Orthop Relat Res. 1967 Sep-Oct;54:163–173. [PubMed] [Google Scholar]
- Thelander L., Reichard P. Reduction of ribonucleotides. Annu Rev Biochem. 1979;48:133–158. doi: 10.1146/annurev.bi.48.070179.001025. [DOI] [PubMed] [Google Scholar]
- Walker D. G. Bone resorption restored in osteopetrotic mice by transplants of normal bone marrow and spleen cells. Science. 1975 Nov 21;190(4216):784–785. doi: 10.1126/science.1105786. [DOI] [PubMed] [Google Scholar]
- YOUNG C. W., HODAS S. HYDROXYUREA: INHIBITORY EFFECT ON DNA METABOLISM. Science. 1964 Nov 27;146(3648):1172–1174. doi: 10.1126/science.146.3648.1172. [DOI] [PubMed] [Google Scholar]
- Yarbro J. W. Further studies on the mechanism of action of hydroxyurea. Cancer Res. 1968 Jun;28(6):1082–1087. [PubMed] [Google Scholar]


