Figure 8. Validation of competition experiment to determine relative affinity.
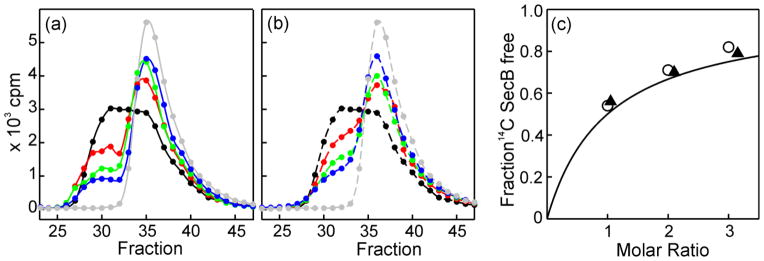
SecA (4 μM dimer) was added either to 14C-SecB (6 μM wild-type tetramer) alone or to 14C-SecB mixed with a nonradioactive competing species of SecB and subjected to size exclusion chromatography. The amount of radioactive SecB in each fraction of eluent was determined. The fractions displayed (23–47) correspond to 7.4 mL to 10.2 mL of eluent. (a) Competition by wild-type SecB. Samples applied to the column contained: SecA mixed with 14C-SecB (6 μM) alone (black); or with competing nonradioactive SecB wild-type at molar ratios of 1(red), 2 (green) or 3 (blue); 14C-SecB (6 μM) without SecA (gray). (b) Competition as described in (a) except that the cross-linked SecB hybrid Aa was the competing SecB species at molar ratios of 1.05 (red), 2.1 (green) and 3.15 (blue). (c) Determination of relative affinity. The fraction of 14C-SecB free was determined by deconvolution of the chromatograms (PeakFit, Systat Software, Inc., San Jose, CA) to resolve the amount of radioactive SecB that was free from that in complex. The continuous line is a theoretical curve generated from the equation, F = R/(A+R) where F is the fraction of SecB free, R the molar ratio of the SecB species, and A, the relative strength of binding, is taken as 1, i.e., equal affinities. Data from competition with wild-type SecB (○) or disulfide cross-linked SecB Aa (▲).
