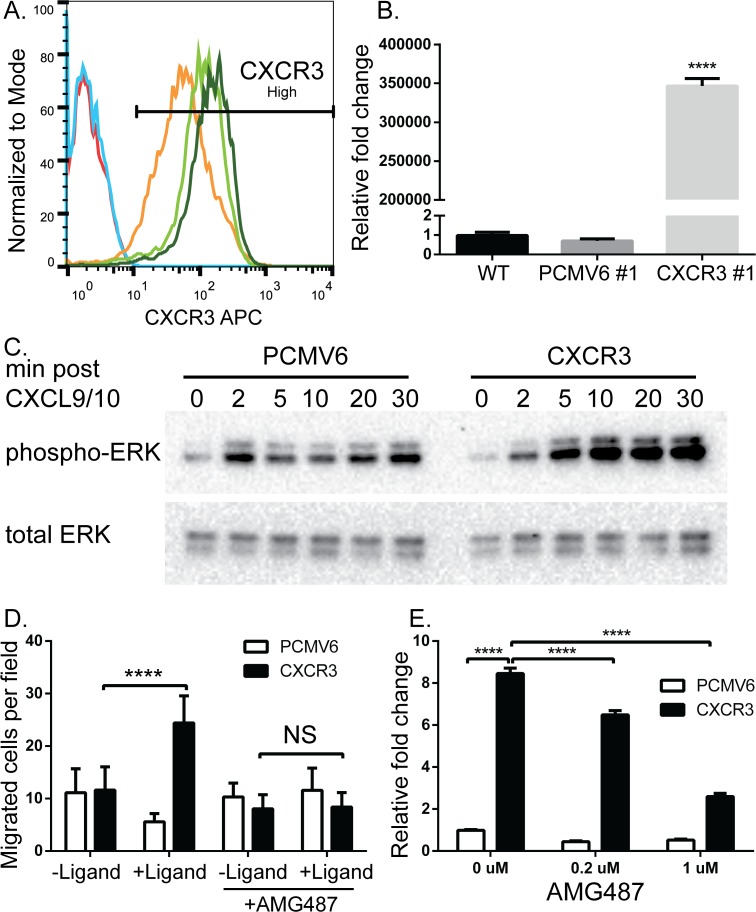
Fig 3. Ectopic overexpression of CXCR3 in BOWES cells increases ligand mediated phosphorylation of ERK, cellular migration, and IL-8 expression.
BOWES cells stably transfected with CXCR3 or an empty vector (PCMV6) were grown in serum-containing media. (A) Flow cytometry and (B) RT-PCR were used to measure CXCR3 expression. (A) BOWES WT (red line), BOWES PCMV6 clone #1(blue line), and BOWES CXCR3 clones #1 (orange line), #2 (light green), and #3 (dark green line). (B) RT-PCR was normalized to CXCR3 clone #2. (C) BOWES PCMV6 and CXCR3 cells were grown in serum-free media for 2 hrs, then in the presence of CXCL9 and CXCL10 for 2, 5, 10, 20, or 30 minutes. Total protein was isolated and probed for pERK and total ERK via immunoblot analysis. (D) BOWES PCMV6 and CXCR3 cells were plated on a membrane above serum-free media with CXCL9/10 (+ligand) or without (-ligand), and total migrated cells per field (40X objective) were quantified after 6hrs. Cells were also plated on the membrane in the presence or absence of AMG487. T-tests were performed on the BOWES CXCR3 migrated cells ± ligand in the presence and absence of AMG487 (NS = P>0.05, **** = P≤ 0.0001). (E) BOWES PCMV6 and CXCR3 cells were grown in serum-containing media for 48 hrs in the presence of ligand, with 0, 0.2, or 1 μM AMG487. Expression of IL-8 was measured via RT-PCR, fold change was calculated relative to BOWES PCMV6 0 μM. T-tests were performed on the relative fold changes, representative data from 3 separate experiments (**** = P≤ 0.0001).