Abstract
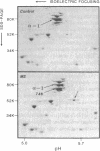
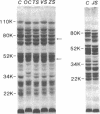
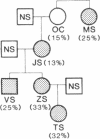
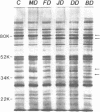
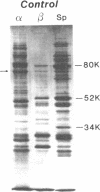
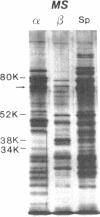
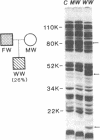
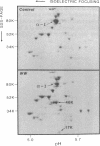
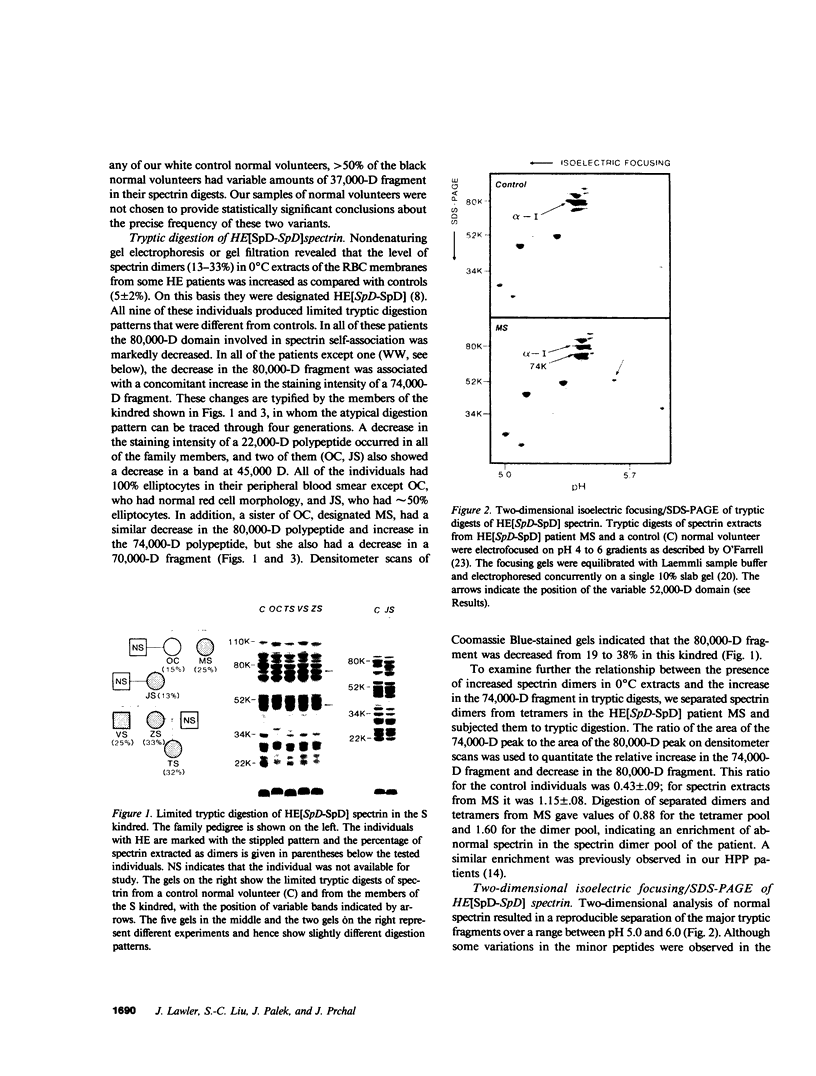
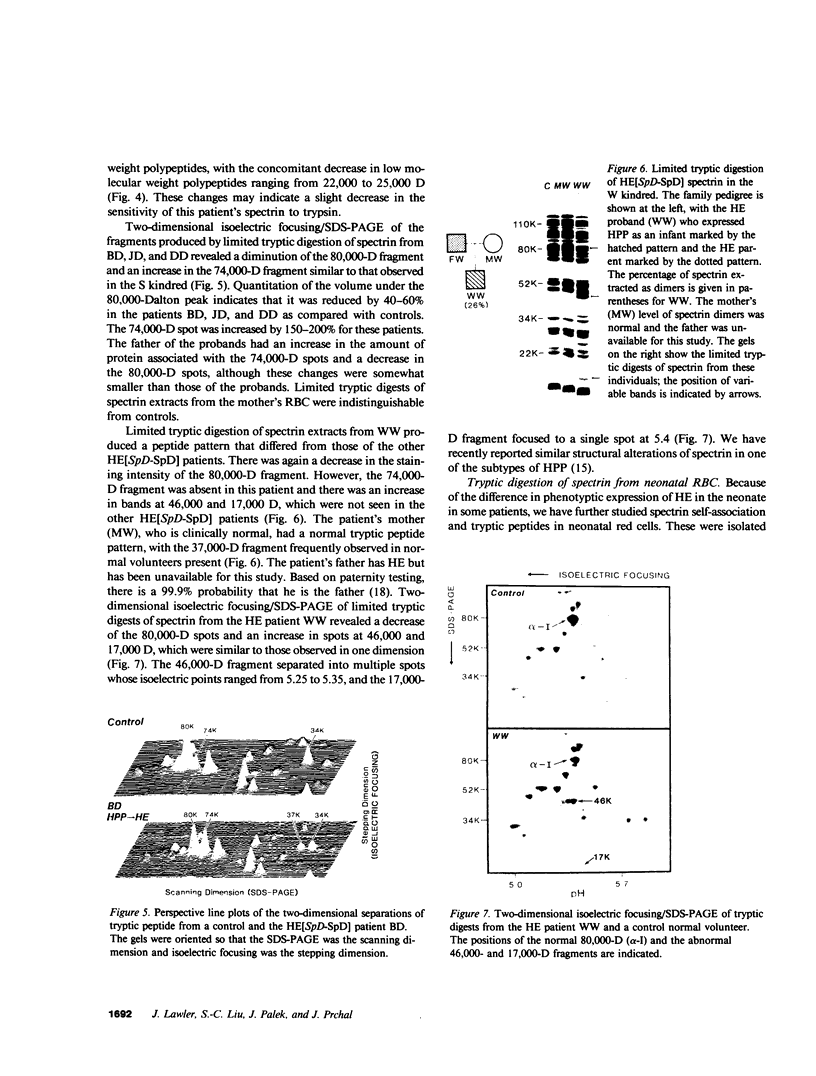
Hereditary elliptocytosis (HE) is a clinically and biochemically heterogenous group of diseases characterized by elliptically shaped erythrocytes and an autosomal dominant mode of inheritance. Whereas the self-association of spectrin heterodimers to tetramers is defective in a subpopulation of HE patients, designated HE[SpD-SpD], it is normal in others. We have examined the peptide pattern produced by limited tryptic digestion of spectrin extracts from patients with HE[SpD-SpD] to determine if the functional defects in spectrin self-association could be correlated with structural changes in the spectrin molecule. Although the peptide pattern produced by limited tryptic digestion of spectrin extracts from those HE patients with normal spectrin self-association was indistinguishable from the pattern from control normal volunteers, digestion of the spectrin extracts from the HE[SpD-SpD] patients showed a reproducible diminution in the 80,000-D domain of the alpha-subunit, which is involved in spectrin dimer self-association. The decrease in the 80,000-D fragment was associated with an increase in a 74,000-D fragment in eight of nine families, or, in one family, with an increase of fragments at 46,000 and 17,000 D. These atypical peptide patterns were similar to those previously reported in two variants of hereditary pyropoikilocytosis (HPP), which also had defective self-association of spectrin. These data indicate that two distinct structural variants of spectrin alpha-subunit are associated with the defective spectrin heterodimer self-association in a subpopulation of HE patients.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Branton D., Cohen C. M., Tyler J. Interaction of cytoskeletal proteins on the human erythrocyte membrane. Cell. 1981 Apr;24(1):24–32. doi: 10.1016/0092-8674(81)90497-9. [DOI] [PubMed] [Google Scholar]
- Coetzer T., Zail S. S. Tryptic digestion of spectrin in variants of hereditary elliptocytosis. J Clin Invest. 1981 May;67(5):1241–1248. doi: 10.1172/JCI110151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- Dhermy D., Lecomte M. C., Garbarz M., Bournier O., Galand C., Gautero H., Feo C., Alloisio N., Delaunay J., Boivin P. Spectrin beta-chain variant associated with hereditary elliptocytosis. J Clin Invest. 1982 Oct;70(4):707–715. doi: 10.1172/JCI110666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J. P., Baines A. J., Hann I. M., Al-Hakim I., Knowles S. M., Hoffbrand A. V. Defective spectrin dimer-dimer association in a family with transfusion dependent homozygous hereditary elliptocytosis. Br J Haematol. 1983 Jun;54(2):163–172. doi: 10.1111/j.1365-2141.1983.tb02085.x. [DOI] [PubMed] [Google Scholar]
- Ji T. H., Kiehm D. J., Middaugh C. R. Presence of spectrin tetramer on the erythrocyte membrane. J Biol Chem. 1980 Apr 10;255(7):2990–2993. [PubMed] [Google Scholar]
- Knowles W. J., Morrow J. S., Speicher D. W., Zarkowsky H. S., Mohandas N., Mentzer W. C., Shohet S. B., Marchesi V. T. Molecular and functional changes in spectrin from patients with hereditary pyropoikilocytosis. J Clin Invest. 1983 Jun;71(6):1867–1877. doi: 10.1172/JCI110942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles W., Marchesi S. L., Marchesi V. T. Spectrin: structure, function, and abnormalities. Semin Hematol. 1983 Jul;20(3):159–174. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lawler J., Liu S. C., Palek J., Prchal J. Molecular defect of spectrin in hereditary pyropoikilocytosis. Alterations in the trypsin-resistant domain involved in spectrin self-association. J Clin Invest. 1982 Nov;70(5):1019–1030. doi: 10.1172/JCI110689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler J., Palek J., Liu S. C., Prchal J., Butler W. M. Molecular heterogeneity of hereditary pyropoikilocytosis: identification of a second variant of the spectrin alpha-subunit. Blood. 1983 Dec;62(6):1182–1189. [PubMed] [Google Scholar]
- Liu S. C., Fairbanks G., Palek J. Spontaneous, reversible protein cross-linking in the human erythrocyte membrane. Temperature and pH dependence. Biochemistry. 1977 Sep 6;16(18):4066–4074. doi: 10.1021/bi00637a020. [DOI] [PubMed] [Google Scholar]
- Liu S. C., Palek J., Prchal J. T. Defective spectrin dimer-dimer association with hereditary elliptocytosis. Proc Natl Acad Sci U S A. 1982 Mar;79(6):2072–2076. doi: 10.1073/pnas.79.6.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S. C., Palek J. Spectrin tetramer-dimer equilibrium and the stability of erythrocyte membrane skeletons. Nature. 1980 Jun 19;285(5766):586–588. doi: 10.1038/285586a0. [DOI] [PubMed] [Google Scholar]
- Lux S. E. Spectrin-actin membrane skeleton of normal and abnormal red blood cells. Semin Hematol. 1979 Jan;16(1):21–51. [PubMed] [Google Scholar]
- Marchesi V. T. The red cell membrane skeleton: recent progress. Blood. 1983 Jan;61(1):1–11. [PubMed] [Google Scholar]
- Morrow J. S., Marchesi V. T. Self-assembly of spectrin oligomers in vitro: a basis for a dynamic cytoskeleton. J Cell Biol. 1981 Feb;88(2):463–468. doi: 10.1083/jcb.88.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Palek J., Lux S. E. Red cell membrane skeletal defects in hereditary and acquired hemolytic anemias. Semin Hematol. 1983 Jul;20(3):189–224. [PubMed] [Google Scholar]
- Prchal J. T., Castleberry R. P., Parmley R. T., Crist W. M., Malluh A. Hereditary pyropoikilocytosis and elliptocytosis: clinical, laboratory, and ultrastructural features in infants and children. Pediatr Res. 1982 Jun;16(6):484–489. doi: 10.1203/00006450-198206000-00017. [DOI] [PubMed] [Google Scholar]
- Speicher D. W., Marchesi V. T. Spectrin domains: proteolytic susceptibility as a probe of protein structure. J Cell Biochem. 1982;18(4):479–492. doi: 10.1002/jcb.1982.240180409. [DOI] [PubMed] [Google Scholar]
- Tchernia G., Mohandas N., Shohet S. B. Deficiency of skeletal membrane protein band 4.1 in homozygous hereditary elliptocytosis. Implications for erythrocyte membrane stability. J Clin Invest. 1981 Aug;68(2):454–460. doi: 10.1172/JCI110275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaselli M. B., John K. M., Lux S. E. Elliptical erythrocyte membrane skeletons and heat-sensitive spectrin in hereditary elliptocytosis. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1911–1915. doi: 10.1073/pnas.78.3.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungewickell E., Gratzer W. Self-association of human spectrin. A thermodynamic and kinetic study. Eur J Biochem. 1978 Aug 1;88(2):379–385. doi: 10.1111/j.1432-1033.1978.tb12459.x. [DOI] [PubMed] [Google Scholar]
- Yurchenco P. D., Speicher D. W., Morrow J. S., Knowles W. J., Marchesi V. T. Monoclonal antibodies as probes of domain structure of the spectrin alpha subunit. J Biol Chem. 1982 Aug 10;257(15):9102–9107. [PubMed] [Google Scholar]
- Zarkowsky H. S. Heat-induced erythrocyte fragmentation in neonatal elliptocytosis. Br J Haematol. 1979 Apr;41(4):515–518. doi: 10.1111/j.1365-2141.1979.tb05889.x. [DOI] [PubMed] [Google Scholar]