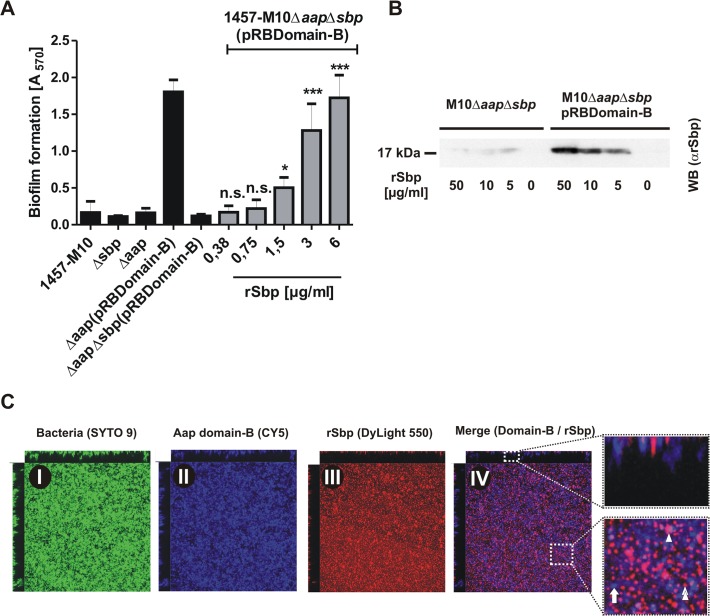
Fig 9. Functional role of Sbp in Aap-mediated S. epidermidis biofilm formation.
(A) Biofilm phenotypes of 1457-M10, 1457-M10Δaap, 1457-M10Δsbp, 1457-M10ΔaapΔsbp, 1457-M10Δaap(pRBDomain-B) and 1457-M10ΔaapΔsbp(pRBDomain-B) were tested in static biofilm assays in the absence (black columns) or presence of varying rSbp concentrations (grey columns). All columns represent mean of 12 values obtained in 3 independent experiments. Error bars represent standard deviations. Significant differences (p< 0.05; one-way ANOVA with Dunnett’s correction for multiple testing) are indicated (*, p<0.05; ***, p<0.001). n.s., not significant. (B) Recruitment of rSbp to the surface of 1457-M10ΔaapΔsbp in the presence of absence of in trans expressed Aap domain-B. Western blot of cell surface protein extracts from identical numbers of bacteria suspended in PBS containing 50, 10, or 5 μg/ml rSbp. PBS without rSbp served as a negative control. rSbp was detected by bioluminescence using a polyclonal rabbit anti-rSbp antiserum and anti-rabbit IgG coupled to peroxidase. (C) Distribution of Aap domain-B and rSbp in living biofilms. 1457-M10ΔaapΔsbp(pRBDomain-B) was grown in the presence of 1.5 μg/ml rSbp-DyLight550. Bacteria were stained with SYTO 9, Aap domain-B was detected using a polyclonal anti-rDomain-B antiserum and a Cy5-labelled anti-rabbit IgG antibody. Panels I–III represent images of each fluorescence channel, image IV is a merge depicting Aap domain-B and rSbp (IV). A zoom-in shows a representative area with Aap domain-B–rSbp co-localizations (purple; double arrow head). Arrow, Aap domain-B expressing cells without Sbp-recruitment (blue); arrow head; Sbp deposition independent from Aap domain-B (red).