The development and approval of new drugs is an arduous and costly task with a high rate of failure, such that recent years have shown little or no increase in successful projects, despite an increasing commitment of resources1. The majority of programs fail for issues unrelated to therapeutic hypothesis, with unexpected clinical side effects and tolerability being crucial issues2,3. Modifications of the standard process to reduce the rate at which compounds fail to progress to approval are required to enhance the efficacy and reduce the resource requirements for drug development. Drug repurposing is one tool to achieve efficiency.
Drug repurposing commonly starts with compounds that have already been tested in humans and have demonstrated an acceptable level of safety and tolerability (Fig. 1). Such compounds are then used for a medical condition other than originally intended. In this way, the development track avoids unexpected derailment due to toxicities not predicted by preclinical work. The issue of efficacy can then be evaluated by clinical trials.
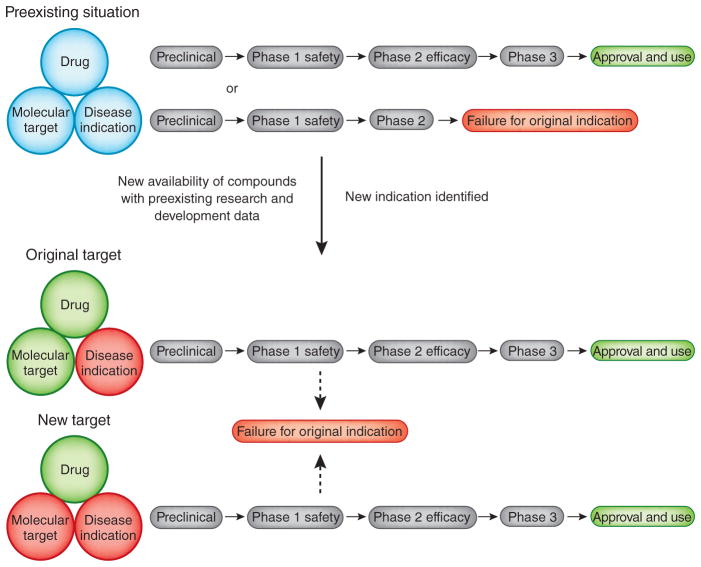
Figure 1.
Repurposing of drugs for rapid development. Existing compounds typically have identified molecular targets and primary indications in which they have either reached approval status or been sidelined somewhere along the pipeline, for efficacy or business reasons. If compounds are made available for further research, then investigation may identify new indications for the same target or new molecular targets with separate indications. A parallel pathway to approval for a repurposed indication can be achieved more robustly by relying on prior safety and tolerability experience with the original indication.
Potential new purposes might be recognized in several ways. Clinical observation in one setting may reveal an unintended second benefit, although this is infrequent. In another pathway, basic discovery research for a particular medical condition may reveal that a particular compound’s molecular target plays a role in a condition other than the original indication. For example, Fyn kinase, targeted by AZD0530, is involved in the mechanism of Alzheimer’s disease as well as in the proliferation of solid tumor cells, broadening AZD0530’s potential utility4 (see below). An alternative scenario is that a particular compound may act via previously unrecognized targets associated with different disease states. For this reason, many academic drug discovery programs search amongst US Food and Drug Administration–approved compounds targeting other molecules as a first step in therapy development. For example, nearly a decade ago, a US National Institutes of Health (NIH)-funded effort identified an action of antibacterial cephalosporins in regulating glutamate transporters5, which spawned a therapeutic trial of ceftriaxone for amyotrophic lateral sclerosis6, though it was halted for lack of efficacy (ClinicalTrials.gov NCT00349622).
In some cases of repurposing, the original intended use of a compound may be successful in its own right, and the repurposing adds an additional indication. For example, the widely used nonsteroidal anti-inflammatory drug ibuprofen was shown to possess a second molecular action: inhibiting the cytoskeletal regulating GTPase RhoA7. This led to preclinical use of ibuprofen in promoting axonal growth after spinal injury8,9, and a clinical trial has now started to assess its potential in traumatic spinal cord injury (ClinicalTrials.gov NCT02096913). However, in other cases, certain compounds never succeeded in their intended application and then are resurrected for new uses. For example, azidothymidine (AZT) was synthesized and considered as an anticancer agent due to its inhibition of oncogenic viruses and tumor cell proliferation10. Although it had no convincing activity in cancer, it inhibited HIV replication and rapidly progressed to approval as an anti-HIV therapy11.
Up until recently, only a limited set of potential compounds have been available for repurposing beyond the specific commercial patent holders for each compound. This is because most compounds with documented preexisting use in humans have intellectual property restrictions. Thus, the use of many compounds potentially available for repurposing is narrowly restricted to programs that fit within the strategic goals of a particular company. Recently, however, both the US National Center for Advancing Translational Sciences and the UK Medical Research Council have recognized the potential for accelerated development that new therapies derived from repurposing of drugs previously tested in humans may hold. The governmental agencies have allied with pharmaceutical companies to make available selected company investigational compounds for clinical testing in government-funded trials by academic investigators. Each selected molecule had undergone considerable research and development by the pharmaceutical industry but had not reached approval status for one reason or another. The inclusion of multiple academic minds to the repurposing of proprietary compounds has been labeled a form of ‘crowdsourcing’ for innovation in drug redirection12. Dozens of projects in which the repurposed efficacy of different compounds is being evaluated are now underway in the United States and the United Kingdom.
One of these projects is focused on the use of AZD0530 for Alzheimer’s disease and is based on the role of Fyn kinase in that disease4. AZD0530 inhibits Src family kinases, including Fyn, and was originally developed for solid tumors13 but showed limited efficacy. AZD0530 is hypothesized now to have greater benefit for slowing the progression of degenerative dementia, and this possibility will be tested in an NIH-supported clinical trial (ClinicalTrials.gov NCT01864655). Another project relates to schizophrenia and the potentially beneficial role of estrogen signaling in treating the disease14. The compound LY500307 is a selective estrogen receptor β agonist initially intended for treatment of benign prostatic hypertrophy15. Because it avoids the full side effects of estrogen itself, it is now being tested for cognitive benefit in schizophrenia (ClinicalTrials.gov NCT01874756). Neither compound would have been available for evaluation in these indications without the NIH’s creation of a commercial-public-academic cooperative project team.
Although the drugs discovered for each unique indication have a popular, academic and intellectual property allure, the development of medical therapy within a confined chemical space of known human drug safety and tolerability has the potential to accelerate the delivery speed for new therapies while reducing biomedical costs. The best chance of identifying new uses for such compounds is by enhancing the availability of such compounds for testing in a broad range of preclinical assays that may identify new indications and/or new targets. Governmental agencies are playing a key role in opening access to largest possible chemical space for which safety data have paved the way to rapid clinical repurposing.
Footnotes
COMPETING FINANCIAL INTERESTS
The author declares no competing financial interests.
References
- 1.Scannell JW, Blanckley A, Boldon H, Warrington B. Nat Rev Drug Discov. 2012;11:191–200. doi: 10.1038/nrd3681. [DOI] [PubMed] [Google Scholar]
- 2.DiMasi JA, Feldman L, Seckler A, Wilson A. Clin Pharmacol Ther. 2010;87:272–277. doi: 10.1038/clpt.2009.295. [DOI] [PubMed] [Google Scholar]
- 3.Arrowsmith J, Miller P. Nat Rev Drug Discov. 2013;12:569. doi: 10.1038/nrd4090. [DOI] [PubMed] [Google Scholar]
- 4.Nygaard HB, van Dyck CH, Strittmatter SM. Alzheimers Res Ther. 2014;6:8. doi: 10.1186/alzrt238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rothstein JD, et al. Nature. 2005;433:73–77. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- 6.Berry JD, et al. PLoS ONE. 2013;8:e61177. doi: 10.1371/journal.pone.0061177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou Y, et al. Science. 2003;302:1215–1217. doi: 10.1126/science.1090154. [DOI] [PubMed] [Google Scholar]
- 8.Wang X, et al. J Neurotrauma. 2009;26:81–95. doi: 10.1089/neu.2007.0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kopp MA, et al. Cell Tissue Res. 2012;349:119–132. doi: 10.1007/s00441-012-1334-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin TS, Prusoff WH. J Med Chem. 1978;21:109–112. [PubMed] [Google Scholar]
- 11.Yarchoan R, Broder S. N Engl J Med. 1987;316:557–564. doi: 10.1056/NEJM198702263160925. [DOI] [PubMed] [Google Scholar]
- 12.Nair P. Proc Natl Acad Sci USA. 2013;110:2430–2432. doi: 10.1073/pnas.201300188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hennequin LF, et al. J Med Chem. 2006;49:6465–6488. doi: 10.1021/jm060434q. [DOI] [PubMed] [Google Scholar]
- 14.Kulkarni J, et al. Schizophr Res. 2001;48:137–144. doi: 10.1016/s0920-9964(00)00088-8. [DOI] [PubMed] [Google Scholar]
- 15.Hilbish KG, Breslin WJ, Johnson JT, Sloter ED. Birth Defects Res B Dev Reprod Toxicol. 2013;98:400–415. doi: 10.1002/bdrb.21083. [DOI] [PubMed] [Google Scholar]