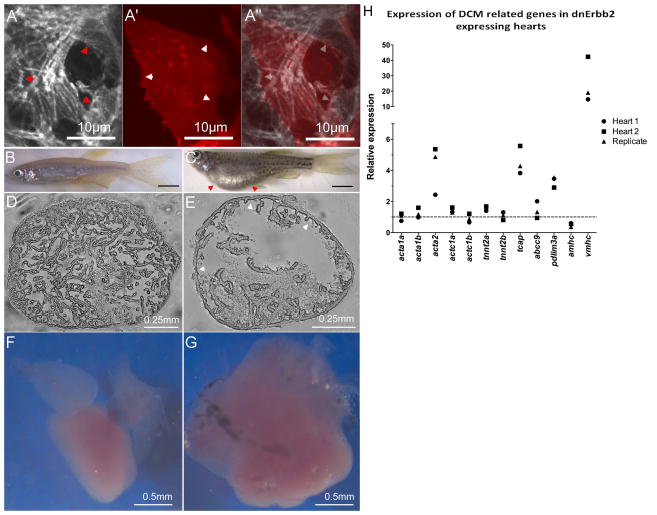
Figure 6. Erbb2 acts cell-autonomously on myofibrillar architecture, and long-term inhibition of Erbb2 signaling causes DCM and dysregulation of DCM genes.
To reduce Erbb2 signaling exclusively in the myocardium, we developed a cardiomyocyte-specific dominant negative strategy. (A–A″) Expression of the dnErbb2 receptor in clones (with coexpression of RFP) reveals cell-autonomous remodeling of myofibrillar architecture, leading to a reduction of thin myofibrils and an overrepresentation of large diameter myofibrils. This effect is most striking at the boundaries between normal and transgenic cells (arrowheads). We further created transgenic animals expressing dnErbb2 under the pan-myocardial myl7 promoter. The resulting transgenics exhibited severe edema (C; red arrowheads), decreased trabeculation (E, white arrowheads) and dilated cardiac morphology (G) compared to non-transgenic siblings (B, D, F). Using quantitative rtPCR, we tested the expression of genes associated with familial cases of DCM (H), and found a strong and consistent upregulation of several members of this gene set including those encoding components of the contractile machinery. The dashed line indicates the expression level in non-transgenic siblings.