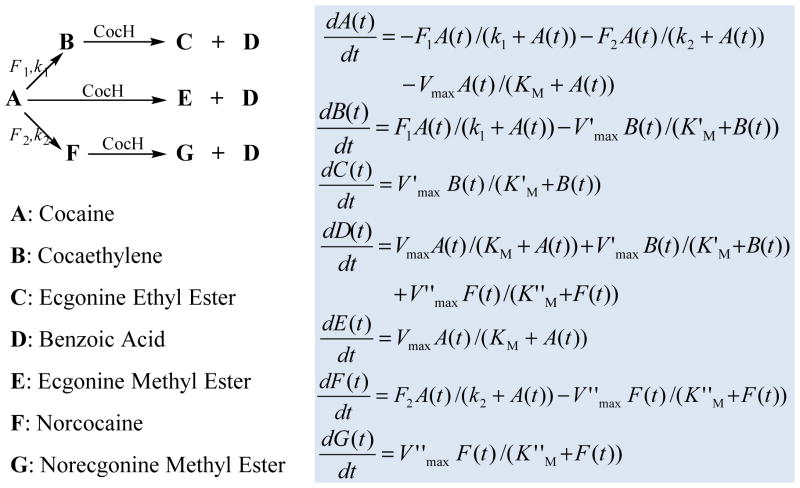Abstract
It is known that majority of cocaine users also consume alcohol. Alcohol can react with cocaine to produce a significantly more cytotoxic compound, cocaethylene. Hence, a truly valuable cocaine-metabolizing enzyme for cocaine abuse/overdose treatment should be efficient for not only cocaine itself, but also cocaethylene. The catalytic parameters (kcat and KM) of human butyrylcholinesterase (BChE) and two mutants (known as cocaine hydrolases E14-3 and E12-7) for cocaethylene have been characterized in the present study, for the first time, in comparison with those for cocaine. Based on the obtained kinetic data, wild-type human BChE has a lower catalytic activity for cocaethylene (kcat = 3.3 min−1, KM = 7.5 μM, and kcat/KM = 4.40 × 105 M−1 min−1) compared to its catalytic activity for (−)-cocaine. E14-3 and E12-7 have a considerably improved catalytic activity against cocaethylene compared to the wild-type BChE. E12-7 is identified as the most efficient enzyme for hydrolyzing cocaethylene in addition to its high activity for (−)-cocaine. E12-7 has an 861-fold improved catalytic efficiency for cocaethylene (kcat = 3600 min−1, KM = 9.5 μM, and kcat/KM = 3.79 × 108 M−1 min−1). It has been demonstrated that E12-7 as an exogenous enzyme can indeed rapidly metabolize cocaethylene in rats. Further kinetic modeling has suggested that E12-7 with an identical concentration as that of the endogenous BChE in human plasma can effectively eliminate (−)-cocaine, cocaethylene, and norcocaine in simplified kinetic models of cocaine abuse and overdose associated with the concurrent use of cocaine and alcohol.
Keywords: Butyrylcholinesterase, protein drug, drug abuse, hydrolysis, kinetics
INTRODUCTION
Cocaine is a widely abused and hepatotoxic drug, without an FDA (U.S. Food and Drug Administration)-approved medication available.[1] Cocaine is a highly addictive and psychoactive stimulant, making it a high priority to develop feasible anti-cocaine medication.[2, 3] The traditional pharmacodynamic approach to cocaine addiction treatment is to develop a small-molecule drug targeting a specific subtype of transporters/receptors and affecting various neurotransmitter systems, such as dopaminergic, serotoninergic, noradrenergic, cholinergic, glutamatergic, GABAergic, and opioidergic pathways, and modulate neurological processes.[4] However, a pharmacodynamic approach to the treatment of cocaine abuse has proven very elusive. The inherent difficulties in antagonizing cocaine in the central nervous system (CNS) have led to the development of alternative approaches to alter the pharmacokinetics of cocaine. In particular, an ideal anti-cocaine medication would accelerate cocaine metabolism,[4] via cocaine hydrolysis at the benzoyl ester,[5–10] because cocaine benzoyl ester hydrolysis generates ecgonine methyl ester (EME) and benzoic acid that are all biologically inactive.
In humans, cocaine is metabolized through hydrolysis catalyzed by plasma enzyme butyrylcholinesterase (BChE) that catalyzes hydrolysis at the benzoyl ester group (Scheme 1), hydrolysis by two liver carboxylesterases (denoted by hCE-1 and hCE-2) that catalyze hydrolysis at the methyl ester and the benzoyl ester, respectively, and oxidation by liver microsomal cytochrome P450 (CYP) 3A4 to produce norcocaine which has similar physiological effects as of cocaine.[10, 11] BChE-catalyzed hydrolysis of cocaine at the benzoyl ester is the primary cocaine-metabolizing pathway which is most suitable for amplification. Unfortunately, wild-type BChE has a low catalytic efficiency against naturally occurring (−)-cocaine (kcat = 4.1 min−1 and KM = 4.5 μM).[12–16]
As an additional challenge to cocaine abuse treatment, statistical data report that the majority of cocaine users (e.g. 92% as of August 2013)[17] also consume alcohol (which always refers to ethanol in this report). Alcohol can react with cocaine under hCE-1 catalysis to produce a significantly more cytotoxic compound, cocaethylene, through transesterification. With alcohol co-administration, ~24% (intravenous), ~34% (oral), or ~18% (smoked) of cocaine is converted to cocaethylene through transesterification.[18] Hence, a truly valuable mutant of human BChE for anti-cocaine enzyme therapy development should be efficient for not only cocaine, but also norcocaine and cocaethylene.
Our computationally designed mutations of human BChE have led to at least 1000-fold improved catalytic efficiency against (−)-cocaine[19–25] and norcocaine.[26] The first one of our designed high-activity mutants of human BChE, i.e. the A199S/S287G/A328W/Y332G mutant,[19] has been recognized as a true cocaine hydrolase (CocH) suitable for testing in humans.[27, 28] The A199S/S287G/A328W/Y332G mutant is currently in double-blind, placebo-controlled clinical trials in humans by Teva Pharmaceutical Industries Ltd for cocaine abuse treatment.[4] Our more recently designed new mutants[22, 24] of human BChE are even more effective against (−)-cocaine. However, it has been unknown whether any of these mutants can also catalyze the hydrolysis of cocaethylene. To our best knowledge, we have not seen a report on the kinetic parameters for cocaethylene hydrolysis catalyzed by wild-type human BChE or any of these BChE mutants. What has been known in literature is that cocaethylene produces more euphoria and possesses a longer half-life than that of cocaine.[18, 29–32]
One might reasonably expect that the BChE mutants with a considerably improved catalytic efficiency against cocaine should also have a considerably improved catalytic efficiency against cocaethylene. However, the recently reported kinetic analysis of the BChE mutants against acetylcholine (ACh), the only known natural substrate of BChE in the body, revealed that the mutations did not improve the catalytic efficiency of BChE against ACh.[25, 33] In fact, the catalytic efficiency of the examined BChE mutants against ACh is slightly lower than that of the wild-type BChE. So, it is unknown whether any of the BChE mutants reported so far has a significantly improved catalytic efficiency against cocaethylene compared to the wild-type BChE.
In the present study, we have characterized the catalytic activity of wild-type human BChE and our discovered A199S/S287G/A328W/Y332G mutant (denoted as E14-3 for convenience) and A199S/F227A/S287G/A328W/Y332G mutant (denoted as E12-7 for convenience) of human BChE against cocaethylene, in comparison with the corresponding catalytic activities against (−)cocaine and norcocaine. The obtained kinetic data have demonstrated that the BChE mutants examined in this study have not only a considerably improved catalytic efficiency against (−)cocaine and norcocaine, but also a considerably improved catalytic efficiency against cocaethylene in vitro and in vivo compared to the wild-type BChE. Further kinetic modeling has demonstrated that these BChE mutants can effectively hydrolyze (−)-cocaine, cocaethylene and norcocaine at the same time in simplified kinetic models of combined cocaine-alcohol abuse.
MATERIALS AND METHODS
Molecular modeling
Cocaethylene binding with human BChE and mutants was modeled by using our previously simulated structures of the same enzymes.[19–26] Our previous molecular dynamics (MD) simulations[25] on the structures of enzyme-cocaine/norcocaine complexes[26] started from the X-ray crystal structure deposited in the Protein Data Bank (pdb code: 1P0P). For each enzyme (human BChE or mutant), cocaethylene was docked into the possible active site of the enzyme by using the AutoDock 4.2 program,[34] as we previously did for the enzyme binding with (−)-cocaine and norcocaine.[26] During the docking process, the Solis and Wets local search method[35] was used for the conformational search and the Lamarkian genetic algorithm (LGA)[34] was employed to deal with the enzyme-ligand interactions. The grid size was set to be 120 × 120 × 120. The finally obtained enzyme-cocaethylene binding structures were the ones with the lowest binding free energies.
Enzyme preparation and in vitro activity assays
Both wild-type and mutants of human BChE were expressed and their enzyme activities against cocaethylene and (−)-cocaine were assayed at the same time under the same experimental conditions so that the activity against cocaethylene can be compared with that against (−)-cocaine for each enzyme. For the purpose of in vitro activity assays, the proteins (wild-type human BChE and mutants) were expressed in human embryonic kidney (HEK) 293F cells. Cells at the density of ~1 × 106 cells/ml were transfected by 293fectin reagent-DNA complexes at the ratio of 2 μl : 1 μg per ml of the cells. Cells were cultured for five more days. The culture medium was harvested, and the protein was purified by using a two-step purification procedure (ion exchange chromatography followed by affinity chromatography), as described previously in detail.[26] The purified protein was dialyzed against phosphate-buffered saline and stored at 4°C or −80°C.
The catalytic activities of the enzymes against cocaethylene and (−)-cocaine were determined by performing a UV-Vis spectrophotometric assay. Using the UV-Vis spectrophotometric assay, the catalytic activities of the enzymes against cocaethylene and (−)-cocaine were determined at the same time under the same experimental conditions. The enzymatic reaction was initiated by adding 180 μl of a substrate (cocaethylene or (−)-cocaine) solution to 20 μl of an enzyme solution. The final initial cocaethylene/(−)-cocaine concentrations were as follows: 100, 50, 20, 10, 5, 2, and 1 μM. The reaction temperature was 25°C, and the buffer used was 0.1 M potassium phosphate (pH 7.4). The initial rates of the enzymatic hydrolysis of cocaethylene/(−)-cocaine in various initial substrate concentrations were estimated by following the change in the intrinsic absorbance peak of cocaethylene/(−)-cocaine at 230 nm (see below for the UV-Vis absorption spectra) with time using a GENios Pro Microplate Reader (TECAN, Research Triangle Park, NC) with the XFluor software. The initial reaction rates were estimated from the linear portions of the progress curves. All assays were carried out in triplicate. The Michaelis-Menten kinetic analysis was performed by using Prism 5 (GraphPad Software Inc., San Diego, CA) to determine the Vmax and KM values.
Subjects for in vivo studies
Male Sprague-Darley rats (200–250 g) were ordered from Harlan (Harlan, Indianapolis, IN) and were housed initially in 2 to 4 rats per cage. All rats were allowed ad libitum access to food and water and were maintained on a 12-hour light and dark cycle with lights on at 8 AM in a room kept at a temperature of 21 to 22°C. Each rat was used only once. Experiments were performed in a same colony room in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the National Institutes of Health. The animal protocol was approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Kentucky.
Characterization of cocaethylene clearance accelerated by E12-7
Cocaethylene (formulated in the salt form of cocaethylene fumarate) was provided by the National Institute on Drug Abuse (NIDA) Drug Supply Program (Bethesda, MD), and the E12-7 material used for in vivo studies in rats were prepared in our recently reported study[36] developing and using stable CHO-S cells. Our in vitro assays revealed that E12-7 expressed in the CHO-S cells had the same catalytic activities of that expressed in HEK 293F cells. General anesthetic isoflurane was utilized with nose cone during the administration of cocaethylene and E12-7 (or saline). Rats were injected with saline or 0.15 mg/kg of E12-7 through the tail vein 1 min before i.v. injection of 3 mg/kg cocaethylene (~7 μmole/kg). Four rats were used for each set of experiments (n=4), About 50 to 75 μl of blood from saphenous veins was collected into capillary tubes and immediately diluted in 100 μl of 250 μM paraoxon at 2, 5, 10, 30, 60, 90, 120, 150, and 180 min after the i.v. injection of cocaethylene. Paraoxon is an irreversible BChE inhibitor that can stop the enzymatic hydrolysis of cocaethylene between sampling and analysis. The diluted blood samples were stored at −70°C and assayed using a High-Performance Liquid Chromatographic (HPLC) method.
Benzoic acid is the product of cocaethylene hydrolysis catalyzed by the enzyme (wild-type BChE or E12-7). The standard benzoic acid for the HPLC analysis was purchased through Sigma Aldrich (Sigma Aldrich, St. Louis, MO). To assay the cocaethylene and benzoic acid concentrations in the blood samples, the frozen whole blood samples were thawed on ice for 3 hours. Then 150 μl of mobile phase (26% acetonitrile and 74% water containing 0.1% TFA) was mixed with each sample, and 50 μl of 10% HClO4 was added to break the blood cell membrane. The mixture was vortexed for 1 min and then centrifuged at 25,000 g for 15 min, and the supernatant was transferred to an autosampler vial of which 200 μl was injected into the chromatographic system. Chromatography was performed using a Waters 1525 binary HPLC pump (Waters Corporation, Milford, MA), a Waters 2487 dual λ absorbance detector, a Waters 2475 multi λ fluorescence detector, and a Waters 717 plus autosampler. The flow rate was 1 ml/min. The eluent was monitored at 230 nm for absorbance of benzoic acid and at 315 nm for fluorescence of cocaethylene while exciting at 230 nm. The cocaethylene peaks appeared at 11.6 min, and the benzoic acid peaks occurred at 12.7 min. The concentrations of cocaethylene and benzoic acid were determined by comparing the corresponding HPLC peak areas with those of authentic standards.
Kinetic modeling
Kinetic modeling of (−)-cocaine in humans was performed by use of a MatLab program (developed in house)[26, 37, 38] in a way similar to that of our recently developed pharmacokinetic modeling of (−)-cocaine in the presence of a cocaine-metabolizing enzyme.[26, 39] The previously used kinetic models did not involve cocaethylene. By using a one-compartment model, the present kinetic modeling also accounted for the transformation of (−)-cocaine to cocaethylene and the subsequent cocaethylene hydrolysis in the presence of a cocaine-metabolizing enzyme. Given in Figure 2 are the reaction scheme and kinetic equations used in the present study. In the kinetic model depicted in Figure 2, the function of alcohol in the transesterification is similar to a co-factor of hCE-1, and the kinetic modeling is based on the assumption that the alcohol concentration is high enough to reach the saturation in which the reaction rate no longer can increase with further increasing the alcohol concentration.
Figure 2.
Reaction scheme and kinetic equations used in the kinetic modeling. X(t) is the concentration of X at time t (X = A to G). Vmax = kcat[E] (for cocaine hydrolysis), V′max = k′cat[E] (for cocaethylene hydrolysis), and V max = k cat[E] (for norcocaine hydrolysis) in which [E] is the concentration of the enzyme (CocH) hydrolyzing all of the three substrates (cocaine, cocaethylene and norcocaine). F1 and k1 represent the kinetic parameters for the transesterification reaction of cocaine with alcohol (catalyzed by hCE-1) to produce cocaethylene when the alcohol concentration is high enough to reach the saturation; in this reaction, the function of alcohol is similar to that of a co-factor. F2 and k2 refer to the kinetic parameters for cocaine oxidation (catalyzed by cytochrome P450 3A4) to norcocaine. V′max, V max, K′M, and K M values used in the modeling were based on the reported overall enzyme activities[50] and the enzyme distributions in the body.[48]
RESULTS AND DISCUSSION
Insights from molecular docking
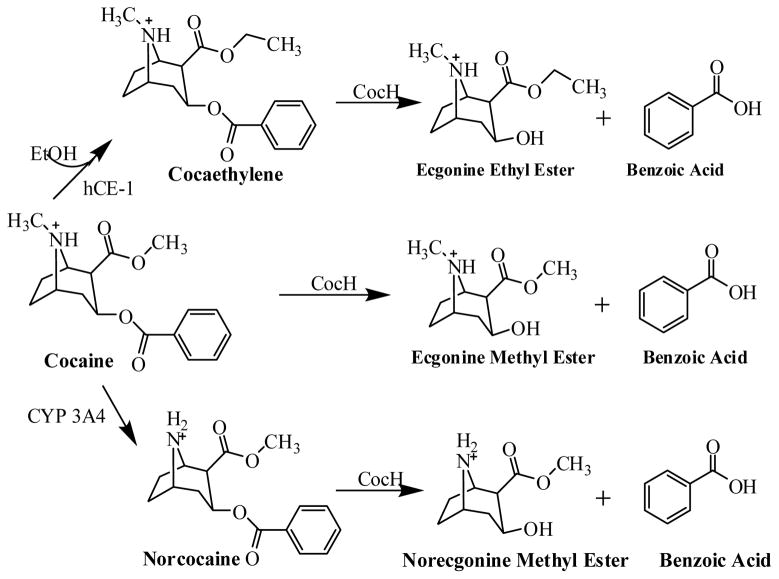
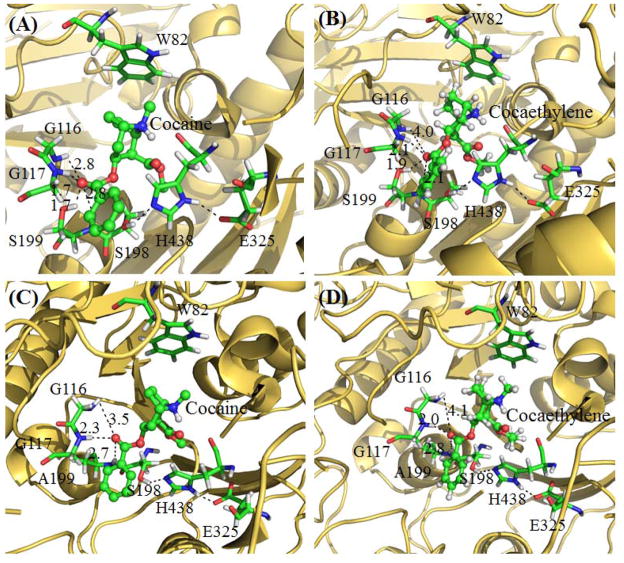
Molecular docking enabled us to understand how cocaethylene may bind with human BChE and the mutants in comparison with (−)-cocaine binding with the same enzymes. As seen in Figure 1, the only structural difference between cocaethylene and (−)-cocaine is that the methyl group on the methyl ester of (−)-cocaine is replaced by an ethyl group in cocaethylene. According to the enzyme-substrate binding structures obtained from molecular docking, the binding mode for each enzyme (wild-type human BChE or its mutant) with cocaethylene is essentially the same as that with (−)-cocaine, particularly for the crucial interactions between the carbonyl oxygen of the substrate and the oxyanion hole (consisting of residues #116, #117, and #199) of the enzyme. The minor structural difference between cocaethylene and (−)-cocaine does not significantly change the binding mode with the BChE or mutant. In particular, there is always only one hydrogen bond between the carbonyl oxygen of the substrate and the oxyanion hole (G117 backbone) of wild-type BChE no matter whether the substrate is cocaethylene or (−)-cocaine, and there are always two hydrogen bonds between the carbonyl oxygen of the substrate and the oxyanion hole (G117 backbone and S199 side chain) of the mutant no matter whether the substrate is cocaethylene or (−)-cocaine. Depicted in Figure 3 are the obtained enzyme-substrate binding structures for cocaethylene and (−)-cocaine with wild-type human BChE and a representative mutant (E12-7). The binding structures with E14-3 (not shown) are similar to those with E12-7 in terms of the overall hydrogen bonding with the oxyanion hole.
Figure 1.
Cocaine metabolites produced in humans through hydrolysis by BChE, oxidation by cytochrome P450 (CYP) 3A4, and reaction of cocaine with alcohol (catalyzed by liver hCE-1).
Figure 3.
Docked structures of the wild-type BChE and CocH3 binding with cocaethylene and (−)-cocaine: (A) Wild-type human BChE binding with (−)-cocaine; (B) Wild-type human BChE binding with cocaethylene; (C) CocH3 binding with (−)-cocaine; (D) CocH3 binding with cocaethylene. Indicated in the figure are the key distances (in Å) of the carbonyl oxygen of the substrate with the hydrogen atoms of the oxyanion hole.
The docking structures depicted in Figure 3 indicate that, regardless of whether the substrate is cocaethylene or (−)-cocaine, the hydroxyl group of S199 side chain in the mutant forms an additional, strong hydrogen bond with the substrate compared to that in the wild-type BChE. Based on this common feature, the same amino-acid mutations that can significantly improve the catalytic efficiency of human BChE against (−)-cocaine may be expected to significantly improve the catalytic efficiency of the enzyme against cocaethylene. Hence, the BChE mutants concerned in the present study are expected to have a significantly improved catalytic efficiency against cocaethylene, although it has been known that these BChE mutants do not have an improved catalytic efficiency against ACh.[25]
Kinetic parameters
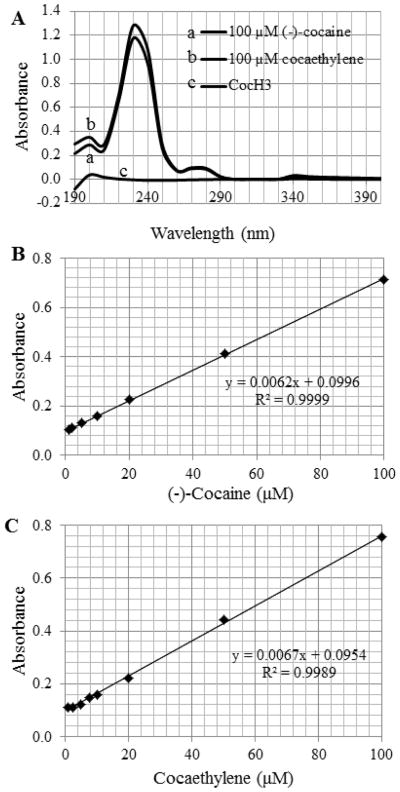
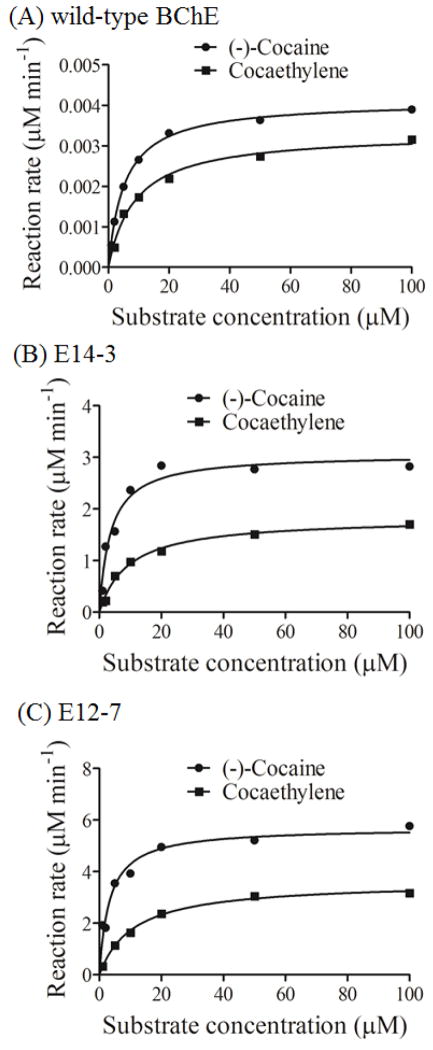
In light of the computational insights, we carried out in vitro experimental tests, including the protein expression and enzyme activity assays, on wild-type human BChE, E14-3, and E12-7. The in vitro assays were based on our observation (Figure 4) that cocaethylene also had an UV-Vis absorption peak at 230 nm as (−)-cocaine, and that the absorption at 230 nm is linearly proportional to the concentration of cocaethylene or (−)-cocaine. The in vitro assays enabled us to determine the catalytic activity of the enzymes against cocaethylene in comparison with the corresponding activity against (−)-cocaine. To minimize the possible systematic experimental errors of the kinetic data, for each enzyme the catalytic activities against both cocaethylene and (−)-cocaine were assayed at the same time under the same experimental conditions so as to reliably determine the catalytic activity of the enzyme against cocaethylene relative to the known activity against (−)-cocaine. Depicted in Figure 5 are the measured kinetic data. Summarized in Table 1 are the kinetic parameters of the enzymes against cocaethylene in comparison with those against (−)-cocaine and norcocaine.
Figure 4.
(A) UV-Vis absorption of (−)-cocaine, cocaethylene, and CocH3. (B) Plot of the absorption at 230 nm versus the concentration of (−)-cocaine. (C) Plot of the absorption at 230 nm versus the concentration of cocaethylene.
Figure 5.
Kinetic data obtained in vitro for enzymatic hydrolysis of (−)-cocaine and cocaethylene: (A) wild-type human BChE; (B) E14-3; (C) E12-7. To minimize the possible systematic experimental errors of the kinetic data, each enzyme’s catalytic activities against both cocaethylene and (−)-cocaine were assayed at the same time under the same experimental conditions so as to reliably determine the kinetic parameters of the enzyme against cocaethylene relative to those against (−)-cocaine. The reaction rate (represented in μM min−1 per nM enzyme) was determined by measuring the rate of the change of the absorbance at 230 nm.
Table 1.
Kinetic parameters determined in vitro for (−)-cocaine, norcocaine and cocaethylene hydrolyses catalyzed by wild-type BChE and its mutants.
| Substrate | Enzymea | KM (μM) | kcat (min−1) | kcat/KM (M−1min−1) | RCEd |
|---|---|---|---|---|---|
| (−)-Cocaineb | WT BChE | 4.5 | 4.1 | 9.11 × 105 | 1 |
| E14-3 | 3.1 | 3,060 | 9.87 × 108 | 1,080 | |
| E12-7 | 3.1 | 5,700 | 1.84 × 109 | 2,020 | |
| Norcocaineb | WT BChE | 15 | 2.8 | 1.87 × 105 | 1 |
| E14-3 | 12 | 766 | 6.38 × 107 | 343 | |
| E12-7 | 13 | 2,610 | 2.01 × 108 | 1,080 | |
| Cocaethylenec | WT BChE | 7.5 | 3.3 | 4.40 × 105 | 1 |
| E14-3 | 8.0 | 1,820 | 2.28 × 108 | 517 | |
| E12-7 | 9.5 | 3,600 | 3.79 × 108 | 861 |
The enzyme under the study was wild-type human BChE (WT BChE), A199S/S287G/A328W/Y332G mutant (E14-3), (CocH2), or A199S/F227A/S287G/A328W/Y332G mutant (E12-7).
Data for wild-type BChE against (−)-cocaine came from reference [12], data for E14-3 against (−)-cocaine came from reference [25], data for CocH3 against (−)-cocaine came from reference [22], and data for all enzymes against norcocaine came from reference [[26]].
All of the kinetic data for cocaethylene were determined in the present study for the first time.
RCE refers to the relative catalytic efficiency (kcat/KM), i.e. the ratio of the kcat/KM value of the mutant to that of wild-type BChE against the same substrate.
A survey of the kinetic parameters summarized in Table 1 reveals that both E14-3 and E12-7 examined in this study have a considerably improved catalytic efficiency (kcat/KM) against cocaethylene. Wild-type BChE has a slightly lower catalytic activity against cocaethylene (kcat = 3.3 min−1, KM = 7.5 μM, and kcat/KM = 4.40 × 105 M−1 min−1) compared to its catalytic activity against (−)-cocaine (kcat = 4.1 min−1, KM = 4.5 μM, and kcat/KM = 9.11 × 105 M−1 min−1). According to the kinetic parameters summarized in Table 1, E14-3 and E12-7 indeed have a significantly improved catalytic efficiency against cocaethylene compared to the wild-type BChE: 517-fold for E14-3 and 861-fold for E12-7. In comparison with the catalytic activities of the same mutant for different substrates, for both E14-3 and E12-7, the catalytic efficiency of the enzyme for cocaethylene is lower than that for (−)-cocaine, but higher than that for norcocaine. Within the enzymes examined in this study, the most efficient BChE mutant (E12-7) against cocaethylene is the same as the most efficient mutant against norcocaine and (−)-cocaine. E12-7 has an 861-fold improved catalytic efficiency against cocaethylene, 1080-fold improved catalytic efficiency against norcocaine, and a 2020-fold improved catalytic efficiency against (−)-cocaine. So, E12-7 is identified as the most promising enzyme (BChE mutant) for metabolizing all of the three toxic substrates: cocaethylene, (−)-cocaine, and norcocaine.
In addition, we also wanted to know whether alcohol has a significant effect on the catalytic activity of the enzymes examined in this study. For this purpose, the catalytic efficiency of E14-3 (as an example) for cocaine hydrolysis was determined in the presence of alcohol (800 mg/dL) in comparison with the corresponding activity in the absence of alcohol. The data depicted in Figure 6 revealed that alcohol (even at the concentration as high as 800 mg/dL or 1.74 M) does not significantly affect the enzyme activity.
Figure 6.
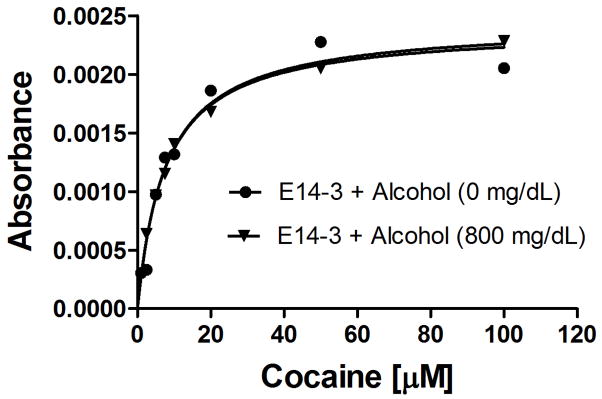
Kinetic data obtained in vitro for E14-3-catalyzed hydrolysis of (−)-cocaine in the presence of alcohol (800 mg/dL or 1.74 M) in comparison with those in the absence of alcohol, showing that alcohol does not significantly modify the catalytic efficiency of the enzyme. The concentrations of the enzyme used for the two experiments were exactly the same. The changes in the UV absorption at 230 nm represent the changes in the cocaine concentrations.
Cocaethylene clearance is accelerated by E12-7
Our recently reported in vivo studies[26, 36] have demonstrated that E12-7 can efficiently metabolize (−)-cocaine and norcocaine in rats. In light of the encouraging in vitro activity data discussed above, we would like to know whether E12-7 can also efficiently metabolize cocaethylene in rats. We characterized the pharmacokinetic profiles of cocaethylene clearance with and without the presence of E12-7 in rats. Four rats (n=4) were injected with saline, followed by i.v. injection of 3 mg/kg cocaethylene. Another set of four rats (n=4) were injected with 0.15 mg/kg E12-7, followed by i.v. injection of 3 mg/kg cocaethylene. The E12-7 dose was 0.15 mg/kg which led to an E12-7 concentration of ~3 mg/L (which is about a half of the average concentration of the endogenous BChE in human, see discussion below) in plasma at ~2 min after the i.v. injection of E12-7 according to our previous study.[36] For each rat, the blood was sampled at 2, 5, 10, 30, 60, 90, 120, 150, and 180 min after the cocaethylene injection. The in vivo data are depicted in Figure 7.
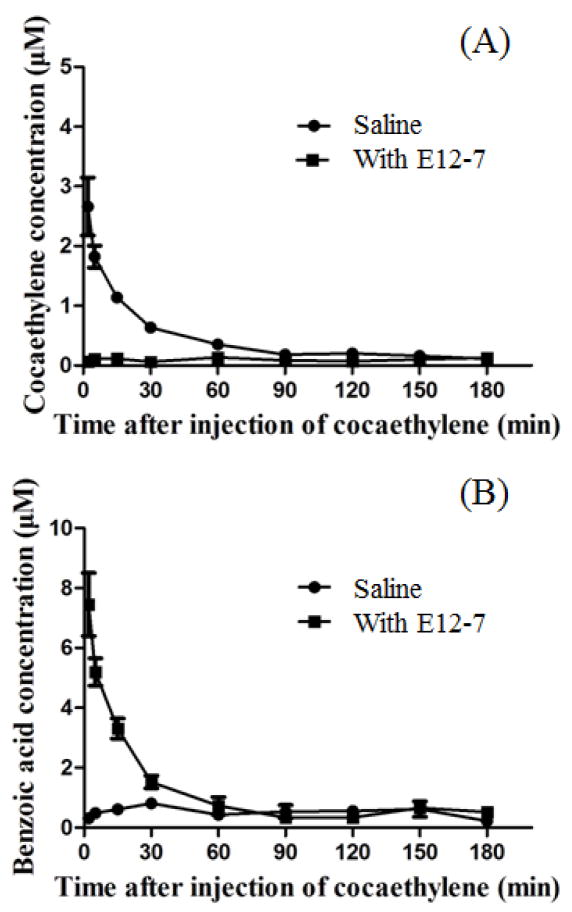
Figure 7.
Cocaethylene clearance (in silico data) accelerated by E12-7: Time-dependent concentrations of cocaethylene (A) and benzoic acid (B) in blood. Benzoic acid is the product of E12-7-catalyzed hydrolysis of cocaethylene. Saline or 0.15 mg/kg E12-7 was injected i.v. in rats (n=4) 1 min before the i.v. injection of 3 mg/kg cocaethylene.
E12-7 can hydrolyze cocaethylene to produce benzoic acid and ecgonine ethyl ester, and greatly accelerate the clearance of cocaethylene from the body. The control curves in Figure 7 reflect the overall effects of all possible cocaethylene elimination pathways.[40] As seen in Figure 7, in the control rats, the average concentration of cocaethylene at the first time point (2 min) was ~2.7 μM, while the average concentration of benzoic acid (metabolite) was ~0.3 μM. In the presence of E12-7, the average concentration of cocaethylene at ~2 min in the blood sample was below the detectable level (~0.1 μM, see Figure 7A), while the average concentration of benzoic acid at the first time point (2 min) was ~7.4 μM (Figure 7B). Most of the cocaethylene was hydrolyzed by E12-7 between the i.v. cocaethylene injection and the first blood sampling at 2 min after the injection. The E12-7-caused dramatic changes in both the cocaethylene and benzoic acid concentrations clearly indicated that cocaethylene was metabolized rapidly to benzoic acid in the presence of E12-7. Notably, as shown in Figure 7B, the benzoic acid concentration in plasma decreased with time. This is because most of the cocaethylene had already been hydrolyzed by E12-7 before the first time point (2 min) so that further generation of benzoic acid after 2 min was negligible compared to the benzoic acid elimination from plasma.
It should be mentioned that the total plasma concentration of cocaethylene and benzoic acid (~7.5 μM) in the presence of E12-7 (when the benzoic acid concentration was higher) was higher than that (3.0 μM) in the absence of E12-7 (when the cocaethylene concentration was higher). This observation might be associated with the potentially different distribution volumes of cocaethylene and benzoic acid in the body. Cocaethylene is an amine drug which can readily cross cell membranes under physiological condition, while benzoic acid primarily exists in the benzoate ion state under the physiological conditions. So, benzoic acid is expected to have a relatively smaller distribution volume compared to cocaethylene.
Effects of E12-7 on the pharmacokinetics of (−)-cocaine and cocaethylene
With E12-7 identified as the most active enzyme (BChE mutant) for cocaethylene in addition to its previously known high catalytic activities for (−)-cocaine and norcocaine, we further carried out kinetic modeling of cocaine metabolism using the kinetic equations shown in Figure 2 in the presence of alcohol and three enzymes: CocH (which refers to either wild-type human BChE or E12-7) in human plasma; hCE-1; and CYP 3A4. Concerning CocH, a typical adult has a blood volume of ~5 L.[39] Previously reported concentrations of endogenous BChE protein in human plasma ranged from 4 to 7 mg/L,[41-43] giving an average value of ~6 mg/L or ~0.07 μM in terms of the total BChE protein concentration (denoted as [E]), assuming that a tetramer of human BChE has four active sites.[44, 45] According to the kinetic data summarized in Table 1, we should have Vmax = 0.29 μM min−1 and KM = 4.5 μM for the wild-type BChE against (−)cocaine, and V′max = 0.23 μM min−1 and K′M = 7.5 μM for the wild-type BChE against cocaethylene, and V″max = 0.20 μM min−1 and K″M = 15 μM for the wild-type BChE against norcocaine, when [E] = 0.07 μM. These kinetic parameters were used in our modeling with the wild-type BChE. Similarly, for E12−7, according to the kinetic data summarized in Table 1, we should have Vmax = 400 μM min−1 and KM = 3.1 μM against (−)-cocaine, V′max = 250 μM min−1 and K′M = 9.5 μM against cocaethylene, and V″max = 180 μM min−1 and K″M = 13 μM against norcocaine, when [E] = 0.07 μM.
For (−)-cocaine transesterification to cocaethylene in the presence of a sufficiently high concentration of alcohol, it has been known that hCE-1 and its (−)-cocaine transesterification-specific activity exist in not only liver, but also other tissues. (−)-Cocaine transesterification-specific activity in isolated kidney microsomes was even greater than that measured in the liver microsomes.[46] It has also been known that (−)-cocaine can diffuse in the body very rapidly to reach the equilibrium.[39] It is reasonable to assume that (−)-cocaine, cocaethylene, and norcocaine distributions in the blood and other tissues can rapidly reach the equilibrium during the metabolic reactions. Thus, it was roughly estimated that F1 = 12.5 μM min−1 and k1 = 0.56 mM for (−)-cocaine transesterification to cocaethylene, according to the available experimental data including the enzyme activity[47] and the enzyme distribution in the body[48] and an assumption that the average hCE-1 density in the whole human body is ~50% of the hCE-1 density in the human liver. Similarly, it was estimated that F2 = 14.4 μM min−1 and k2 = 2.7 mM for the enzymatic oxidation of (−)-cocaine to norcocaine.[26] These roughly estimated kinetic parameters were used in our kinetic modeling with various initial concentrations; our additional modeling tests revealed that kinetic modeling using different values of the catalytic parameters would lead to the same qualitative conclusions mentioned below.
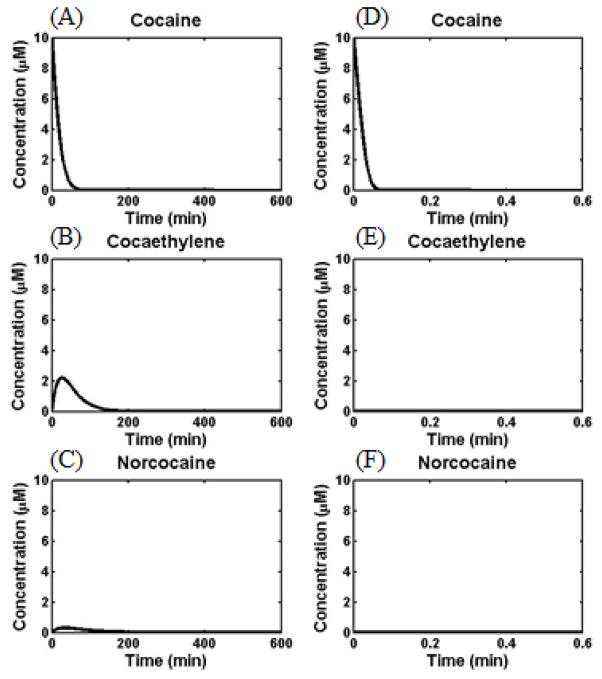
The first set of initial concentrations used in the kinetic modeling include: A(0) (the initial concentration of (−)-cocaine) = 1 to 100 μM while B(0) (the initial concentration of cocaethylene) = 0 and F(0) (the initial concentration of norcocaine) = 0. Depicted in Figure 8 are the time-dependent concentrations of (−)-cocaine, cocaethylene and norcocaine when A(0) = 10 μM in the presence of alcohol, hCE-1, CYP 3A4 and wild-type human BChE (Figure 8A to C) or alcohol, hCE-1, CYP 3A4 and E12-7 (Figure 8D to F). As seen in Figure 8A to C, in the presence of alcohol, hCE-1, CYP 3A4 and wild-type human BChE (without administration of any exogenous enzyme), (−)-cocaine has an area under the curve (AUC) of 178 μM·min and a half-life (t1/2) of 14 min, cocaethylene has an AUC of 152 μM·min and a half-life of 67 min, and norcocaine has an AUC of 37 μM·min and a half-life of 99 min. The modeling data suggest that cocaethylene can exist in the body for a much longer time compared to (−)-cocaine itself because the endogenous wild-type BChE has a relatively lower catalytic activity against cocaethylene.
Figure 8.
The modeled concentrations (in silico data) of (−)-cocaine (A), cocaethylene (B), and norcocaine (C) in human blood when the initial (−)-cocaine concentration is 10 μM in the presence of alcohol, hCE-1, CYP 3A4 and wild-type human BChE, and concentrations of (−)cocaine (D), cocaethylene (E), and norcocaine (F) in human blood when the initial (−)-cocaine concentration is 10 μM in the presence of alcohol, hCE-1, CYP 3A4 and E12-7.
Based on the kinetic modeling, cumulatively, about 39% of (−)-cocaine has been metabolized to cocaethylene and then ecgonine ethyl ester and benzoic acid when A(0) = 10 μM. Further, the modeling data summarized in Table 2 indicate that percentage contribution of (−)cocaine transesterification to cocaethylene, as well as the AUC and t1/2 of (−)-cocaine, cocaethylene and norcocaine, should increase with increasing the initial (−)-cocaine concentration. The data of the kinetic modeling are in reasonable agreement with the experimental observations that ~18% to ~34% of cocaine is converted to cocaethylene through transesterification when the peak concentration of cocaine in human plasma is 1 to 2 μM. According to the modeling data in Table 2, when A(0) = 1 or 2 μM, about 27% or 29% of (−)cocaine has been converted to cocaethylene and then ecgonine ethyl ester and benzoic acid.
Table 2.
Kinetic parameters obtained from in silico modeling for (−)-cocaine and norcocaine hydrolyses catalyzed by wild-type human BChE and its mutants. A(0) is the initial concentration of (−)-cocaine. Coca% refers to the percentage contribution of (−)-cocaine metabolism through hCE-1-catalyzed transesterification to cocaethylene. Coc, Coca, and Norc represent (−)-cocaine, cocaethylene, and norcocaine, respectively.
| A(0) (μM) | hCE-1, CYP 3A4, and wild-type BChE | hCE-1, CYP 3A4, and E12-7 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AUC(μM·min) | t1/2 (min)b | Coca% | AUC(μM·min) | t1/2 (min)b | Coca% | |||||||||
| Coc | Coca | Norc | Coc | Coca | Norc | Coc | Coca | Norc | Coc | Coca | Norc | |||
| 1 | 12 | 8.9 | 2.5 | 8.5 | 54 | 91 | 27% | 0.009 | 0.000 | 0.000 | 0.007 | 0.051 | 0.078 | 0.02% |
| 2 | 26 | 19 | 5.3 | 9.3 | 56 | 92 | 29% | 0.021 | 0.000 | 0.000 | 0.008 | 0.053 | 0.079 | 0.03% |
| 3 | 41 | 31 | 8.4 | 10 | 57 | 93 | 30% | 0.035 | 0.000 | 0.000 | 0.009 | 0.054 | 0.081 | 0.03% |
| 4 | 58 | 44 | 12 | 11 | 59 | 94 | 32% | 0.051 | 0.000 | 0.000 | 0.010 | 0.056 | 0.083 | 0.03% |
| 5 | 75 | 59 | 15 | 11 | 60 | 95 | 33% | 0.070 | 0.000 | 0.000 | 0.011 | 0.057 | 0.084 | 0.03% |
| 6 | 94 | 74 | 19 | 12 | 62 | 96 | 35% | 0.092 | 0.000 | 0.000 | 0.013 | 0.058 | 0.086 | 0.03% |
| 7 | 114 | 92 | 23 | 13 | 63 | 97 | 36% | 0.116 | 0.000 | 0.000 | 0.014 | 0.060 | 0.088 | 0.04% |
| 8 | 134 | 110 | 28 | 13 | 64 | 98 | 37% | 0.142 | 0.000 | 0.000 | 0.015 | 0.062 | 0.089 | 0.04% |
| 9 | 155 | 130 | 32 | 14 | 66 | 99 | 38% | 0.171 | 0.000 | 0.000 | 0.016 | 0.064 | 0.091 | 0.04% |
| 10 | 178 | 152 | 37 | 14 | 67 | 99 | 39% | 0.203 | 0.000 | 0.000 | 0.018 | 0.066 | 0.093 | 0.05% |
| 20 | 432 | 451 | 91 | 17 | 83 | 107 | 47% | 0.656 | 0.001 | 0.000 | 0.030 | 0.083 | 0.111 | 0.07% |
| 30 | 728 | 925 | 156 | 19 | 100 | 114 | 53% | 1.359 | 0.001 | 0.000 | 0.042 | 0.100 | 0.129 | 0.10% |
| 40 | 1051 | 1599 | 229 | 21 | 117 | 119 | 56% | 2.312 | 0.002 | 0.000 | 0.055 | 0.117 | 0.147 | 0.12% |
| 50 | 1395 | 2495 | 310 | 22 | 134 | 124 | 59% | 3.514 | 0.003 | 0.001 | 0.068 | 0.134 | 0.165 | 0.15% |
| 60 | 1755 | 3627 | 398 | 23 | 151 | 129 | 62% | 4.965 | 0.004 | 0.001 | 0.079 | 0.150 | 0.183 | 0.17% |
| 70 | 2129 | 5007 | 493 | 24 | 169 | 133 | 64% | 6.665 | 0.005 | 0.001 | 0.093 | 0.166 | 0.200 | 0.20% |
| 80 | 2515 | 6642 | 594 | 25 | 187 | 137 | 65% | 8.614 | 0.007 | 0.002 | 0.104 | 0.182 | 0.217 | 0.22% |
| 90 | 2912 | 8540 | 702 | 26 | 206 | 141 | 66% | 10.81 | 0.008 | 0.002 | 0.117 | 0.198 | 0.234 | 0.24% |
| 100 | 3319 | 10705 | 817 | 26 | 224 | 145 | 68% | 13.26 | 0.010 | 0.002 | 0.130 | 0.213 | 0.250 | 0.27% |
In the presence of alcohol, hCE-1, CYP 3A4 and E12-7 (exogenous enzyme with [E] = 0.07 μM), when A(0) = 10 μM, (−)-cocaine only has an AUC of 0.203 μM·min and a half-life of 0.018 min, and both cocaethylene and norcocaine have the AUC values smaller than 0.001 μM·min, as shown in Figure 8D to F. Both the AUC and t1/2 values are all negligible when E12-7 is administered as an exogenous enzyme (or provided via gene therapy[49]) with the E12-7 concentration being the same as that of the endogenous BChE ([E] = 0.07 μM). As seen in Table 2, for all of the three toxic compounds, i.e. (−)-cocaine, cocaethylene and norcocaine, the AUC and t1/2 increase with increasing the initial (−)-cocaine concentration, but not dramatically. In particular, even if A(0) = 100 μM, the half-life of (−)-cocaine is still only 0.130 min, and the half-life of cocaethylene is still only 0.213 min in the presence of E12-7. Clearly, all of the three toxic compounds can be eliminated effectively and rapidly at the same time when E12-7 is administered as an exogenous enzyme with the E12-7 concentration being the same as that of the endogenous BChE ([E] = 0.07 μM) in the simplified kinetic model.
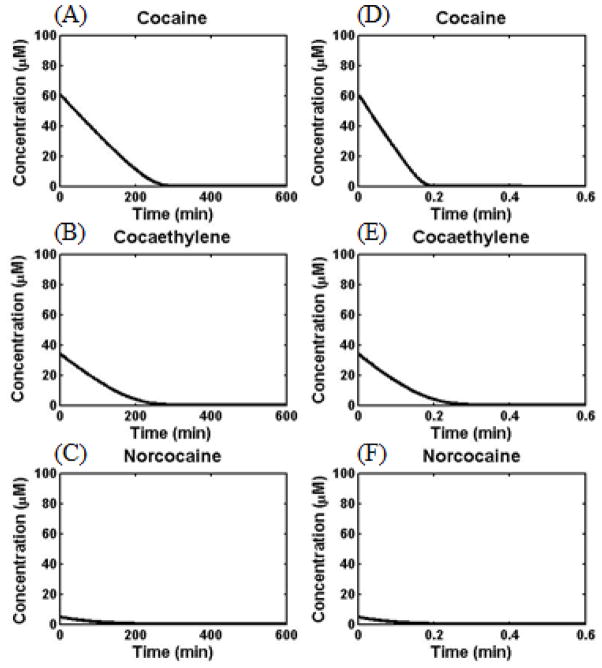
The initial concentrations used in the above kinetic modeling may represent the possible cocaine abuse treatment conditions in which E12-7 is administered prior to the cocaine administration. For possible cocaine overdose treatment using an exogenous cocaine-metabolizing enzyme, the cocaine abusers have already taken cocaine and alcohol, and converted some cocaine to cocaethylene and norcocaine before the enzyme administration. In order to know whether E12-7 is also efficacious in hydrolysis of cocaethylene, in addition to (−)-cocaine and norcocaine, for the cocaine overdose treatment, we performed an additional, simplified kinetic modeling by assuming that 34% (−)-cocaine has been converted to cocaethylene, 5% (−)cocaine has been converted to norcocaine, and 61% (−)-cocaine remains as (−)-cocaine when t = 0. Depicted in Figure 9 are data obtained from the simplified kinetic modeling when A(0) = 61 μM, B(0) = 34 μM, and F(0) = 5 μM. As seen in Figure 8A to C, in the absence of E12-7, (−)-cocaine and cocaethylene may last in the body for a long time (>200 min). As seen in Figure 8D to F, in the presence of 0.07 μM E12-7, all of the three toxic compounds may be eliminated completely in ~0.2 min. These data qualitatively suggest that E12-7 should be effective for cocaine overdose treatment even in the case of combined use of cocaine and alcohol.
Figure 9.
The modeled concentrations (in silico data) of (−)-cocaine, cocaethylene, and norcocaine in human blood when the initial concentrations of (−)-cocaine, cocaethylene, and norcocaine are 61, 34, and 5 μM, respectively. (A) to (C) refer to the time-dependent concentrations in the presence of wild-type human BChE (without E12-7), whereas (D) to (F) refer to the time-dependent concentrations in the presence of 0.07 μM E12-7.
CONCLUSION
The catalytic activity of human BChE and two BChE mutants (known as cocaine hydrolases E14-3 and E12-7) for cocaethylene has been characterized in comparison with the corresponding catalytic activity for cocaine. The kinetic data reveal that wild-type human BChE has a relatively lower catalytic activity against cocaethylene (kcat = 3.3 min−1, KM = 7.5 μM, and kcat/KM = 4.40 × 105 M−1 min−1) compared to its catalytic activity against (−)-cocaine (kcat = 4.1 min−1, KM = 4.5 μM, and kcat/KM = 9.11 × 105 M−1 min−1). It has been shown that E14-3 and E12-7 have not only a considerably improved catalytic efficiency against cocaine and norcocaine, but also a considerably improved catalytic efficiency against cocaethylene, compared to the wild-type BChE. The most efficient enzyme (E12-7, i.e. the A199S/F227A/S287G/A328W/Y332G mutant of human BChE) against cocaethylene is the same as the most efficient one against norcocaine in addition to the known high catalytic activity against (−)-cocaine. E12-7 has an 861-fold improved catalytic efficiency against cocaethylene (kcat = 3600 min−1, KM = 9.5 μM, and kcat/KM = 3.79 × 108 M−1 min−1). Thus, E12-7 is identified as the most promising enzyme for hydrolyzing for all three toxic compounds, i.e. (−)-cocaine, cocaethylene and norcocaine. It has been demonstrated that E12-7 as an exogenous enzyme can indeed rapidly metabolize cocaethylene, in addition to cocaine and norcocaine, in rats. Further kinetic modeling has suggested that E12-7 with a concentration similar to that of the endogenous BChE in human plasma can effectively eliminate all of the three toxic compounds in simplified kinetic models of cocaine abuse and overdose associated with the concurrent use of cocaine and alcohol.
Acknowledgments
We thank the Computer Center at the University of Kentucky for supercomputing time on a Dell X-series Cluster with 384 nodes or 4,768 processors.
FUNDING
This work was supported by the National Institutes of Health (NIH) (grant numbers R01 DA035552, R01 DA032910, R01 DA013930, and R01 DA025100). M.Z. thanks the National Institute on Drug Abuse (NIDA) of the NIH for a scholarship award [number 3R01DA032910-02S1] from the 2013 Summer Research with NIDA Program and the Kentucky Young Researchers Program (KYRP) for a research grant.
Footnotes
AUTHOR CONTRIBUTION
Shurong Hou prepared the enzymes, carried out the in vivo tests, and trained Max Zhan to perform the in vitro activity assays. Max Zhan performed the molecular docking, in vitro activity assays, and kinetic modeling. Xirong Zheng assisted Shurong Hou to perform the in vivo studies. Both Shurong Hou and Max Zhan contributed to the manuscript preparation. Fang Zheng and Chang-Guo Zhan designed the project and finalized the paper.
References
- 1.UNODC. World Drug Report 2010. United Nations Publication; 2010. Sales No. E.10.XI.13) ed.)^eds.) [Google Scholar]
- 2.Karila L, Gorelick D, Weinstein A, Noble F, Benyamina A, Coscas S, Blecha L, Lowenstein W, Martinot JL, Reynaud M, Lépine JP. New treatments for cocaine dependence: a focused review. Intern J Neuropsychopharmacol. 2008;11:425–438. doi: 10.1017/S1461145707008097. [DOI] [PubMed] [Google Scholar]
- 3.Xi ZX, Gardner LE. Hypothesis-driven medication discovery for the treatment of psychostimulant addiction. Curr Drug Abuse Rev. 2008;1:303–327. doi: 10.2174/1874473710801030303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zheng F, Zhan CG. Are pharmacokinetic approaches feasible for treatment of cocaine addiction and overdose? Future Med Chem. 2012;4:125–128. doi: 10.4155/fmc.11.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meijler MM, Kaufmann GF, Qi L, Mee JM, Coyle AR, Moss JA, Wirsching P, Matsushita M, Janda KD. Fluorescent Cocaine Probes:A Tool for the Selection and Engineering of Therapeutic Antibodies. J Am Chem Soc. 2005;127:2477–2484. doi: 10.1021/ja043935e. [DOI] [PubMed] [Google Scholar]
- 6.Carrera MRA, Kaufmann GF, Mee JM, Meijler MM, Koob GF, Janda KD. Treating cocaine addiction with viruses. Proc Natl Acad Sci USA. 2004;101:10416–10421. doi: 10.1073/pnas.0403795101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Landry DW, Zhao K, Yang GX, Glickman M, Georgiadis TM. Antibody-catalyzed degradation of cocaine. Science. 1993;259:1899–1901. doi: 10.1126/science.8456315. [DOI] [PubMed] [Google Scholar]
- 8.Zhan CG, Deng SX, Skiba JG, Hayes BA, Tschampel SM, Shields GC, Landry DW. First-principle studies of intermolecular and intramolecular catalysis of protonated cocaine. J Comput Chem. 2005;26:980–986. doi: 10.1002/jcc.20241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kamendulis LM, Brzezinski MR, Pindel EV, Bosron WF, Dean RA. Metabolism of cocaine and heroin is catalyzed by the same human liver carboxylesterases. J Pharmacol Exp Ther. 1996;279:713–717. [PubMed] [Google Scholar]
- 10.Gorelick DA. Pharmacokinetic approaches to treatment of drug addiction. Expert Rev Clin Pharmacol. 2008;1:277–290. doi: 10.1586/17512433.1.2.277. [DOI] [PubMed] [Google Scholar]
- 11.Gorelick DA. Enhancing cocaine metabolism with butyrylcholinesterase as a treatment strategy. Drug Alcohol Depend. 1997;48:159–165. doi: 10.1016/s0376-8716(97)00119-1. [DOI] [PubMed] [Google Scholar]
- 12.Sun H, Pang YP, Lockridge O, Brimijoin S. Re-engineering Butyrylcholinesterase as a Cocaine Hydrolase. Mol Pharmacol. 2002;62:220–224. doi: 10.1124/mol.62.2.220. [DOI] [PubMed] [Google Scholar]
- 13.Hamza A, Cho H, Tai HH, Zhan CG. Molecular Dynamics Simulation of Cocaine Binding with Human Butyrylcholinesterase and Its Mutants. J Phys Chem B. 2005;109:4776–4782. doi: 10.1021/jp0447136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gatley SJ. Activities of the enantiomers of cocaine and some related compounds as substrates and inhibitors of plasma butyrylcholinesterase. Biochem Pharmacol. 1991;41:1249–1254. doi: 10.1016/0006-2952(91)90665-r. [DOI] [PubMed] [Google Scholar]
- 15.Darvesh S, Hopkins DA, Geula C. Neurobiology of butyrylcholinesterase. Nat Rev Neurosci. 2003;4:131–138. doi: 10.1038/nrn1035. [DOI] [PubMed] [Google Scholar]
- 16.Giacobini E. Butyrylcholinesterase: Its Function and Inhibitors. Martin Dunitz, an imprint of the Taylor and Francis Group plc; London: 2003. [Google Scholar]
- 17.Gorelick DA, Saxon AJ, Hermann R. Cocaine use disorder in adults: Epidemiology, pharmacology, clinical manifestations, medical consequences, and diagnosis. 2013 http://www.uptodate.com/contents/cocaine-use-disorder-in-adults-epidemiology-pharmacology-clinical-manifestations-medical-consequences-and-diagnosis (updated: Aug 15, 2013)
- 18.Herbst ED, Harris DS, Everhart ET, Mendelson J, Jacob P, Jones RT. Cocaethylene formation following ethanol and cocaine administration by different routes. Exp Clin Psychopharmacol. 2011;19:95–104. doi: 10.1037/a0022950. [DOI] [PubMed] [Google Scholar]
- 19.Pan Y, Gao D, Yang W, Cho H, Yang G, Tai HH, Zhan CG. Computational redesign of human butyrylcholinesterase for anticocaine medication. Proc Natl Acad Sci USA. 2005;102:16656–16661. doi: 10.1073/pnas.0507332102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pan Y, Gao D, Yang W, Cho H, Zhan CG. Free Energy Perturbation (FEP) Simulation on the Transition States of Cocaine Hydrolysis Catalyzed by Human Butyrylcholinesterase and Its Mutants. J Am Chem Soc. 2007;129:13537–13543. doi: 10.1021/ja073724k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pan Y, Gao D, Zhan CG. Modeling the Catalysis of Anti-Cocaine Catalytic Antibody: Competing Reaction Pathways and Free Energy Barriers. J Am Chem Soc. 2008;130:5140–5149. doi: 10.1021/ja077972s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng F, Yang W, Ko MC, Liu J, Cho H, Gao D, Tong M, Tai HH, Woods JH, Zhan CG. Most Efficient Cocaine Hydrolase Designed by Virtual Screening of Transition States. J Am Chem Soc. 2008;130:12148–12155. doi: 10.1021/ja803646t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang W, Pan Y, Zheng F, Cho H, Tai HH, Zhan CG. Free-Energy Perturbation Simulation on Transition States and Redesign of Butyrylcholinesterase. Biophys J. 2009;96:1931–1938. doi: 10.1016/j.bpj.2008.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng F, Yang W, Xue L, Hou S, Liu J, Zhan CG. Design of high-activity mutants of human butyrylcholinesterase against (−)-cocaine: structural and energetic factors affecting the catalytic efficiency. Biochemistry. 2010;49:9113–9119. doi: 10.1021/bi1011628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xue L, Ko MC, Tong M, Yang W, Hou S, Fang L, Liu J, Zheng F, Woods JH, Tai HH, Zhan CG. Design, preparation, and characterization of high-activity mutants of human butyrylcholinesterase specific for detoxification of cocaine. Mol Pharmacol. 2011;79:290–297. doi: 10.1124/mol.110.068494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhan M, Hou S, Zhan CG, Zheng F. Kinetic characterization of high-activity mutants of human butyrylcholinesterase for the cocaine metabolite norcocaine. Biochem J. 2014;457:197–206. doi: 10.1042/BJ20131100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brimijoin S, Gao Y, Anker JJ, Gliddon LA, LaFleur D, Shah R, Zhao Q, Singh M, Carroll ME. A Cocaine Hydrolase Engineered from Human Butyrylcholinesterase Selectively Blocks Cocaine Toxicity and Reinstatement of Drug Seeking in Rats. Neuropsychopharmacology. 2008;33:2715–2725. doi: 10.1038/sj.npp.1301666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anker JJ, Brimijoin S, Gao Y, Geng L, Zlebnik NE, Parks RJ, Carroll ME. Cocaine hydrolase encoded in viral vector blocks the reinstatement of cocaine seeking in rats for 6 months. Biol Psychiatry. 2012;71:700–705. doi: 10.1016/j.biopsych.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farre M, Torre R, Llorente M, Lamas X, Ugena B, Segura J, Cami J. Alcohol and cocaine interactions in humans. J Pharmacol Exp Ther. 1993;266:1364–1373. [PubMed] [Google Scholar]
- 30.Laizure SC, Mandrell T, Gades NM, Parker RB. Cocaethylene metabolism and interaction with cocaine and ethanol: role of carboxylesterases. Drug Metabol Disposit. 2003;31:16–20. doi: 10.1124/dmd.31.1.16. [DOI] [PubMed] [Google Scholar]
- 31.Pan HT, Menacherry S, Justice JB., Jr Differences in the pharmacokinetics of cocaine in naive and cocaine-experienced rats. J Neurochem. 1991;56:1299–1306. doi: 10.1111/j.1471-4159.1991.tb11425.x. [DOI] [PubMed] [Google Scholar]
- 32.Pan WJ, Hedaya MA. Cocaine and alcohol interactions in the rat: effect on cocaine pharmacokinetics and pharmacodynamics. J Pharm Sci. 1999;88:459–467. doi: 10.1021/js980282p. [DOI] [PubMed] [Google Scholar]
- 33.Hou S, Xue L, Yang W, Fang L, Zheng F, Zhan CG. Substrate selectivity of high-activity mutants of human butyrylcholinesterase. Org Biomol Chem. 2013;11:7477–7485. doi: 10.1039/c3ob41713a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morris GM, Goodsell DS, Halliday RS, Huey R, Hart WE, Belew RK, Olson AJ. Automated docking using a Lamarckian genetic algorithm and empirical binding free energy function. J Comput Chem. 1998;19:1639–1662. [Google Scholar]
- 35.Solis FJ, Wets RJB. Minimization by random search techniques. Maths Opera Res. 1981;6:19–30. [Google Scholar]
- 36.Xue L, Hou S, Tong M, Fang L, Chen X, Jin Z, Tai HH, Zheng F, Zhan CG. Preparation and in vivo characterization of a cocaine hydrolase engineered from human butyrylcholinesterase for metabolizing cocaine. Biochem J. 2013;453:447–454. doi: 10.1042/BJ20130549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller JH, Zheng F, Jin S, Opresko LK, Wiley HS, Resat H. A Model of Cytokine Shedding Induced by Low Doses of Gamma Radiation. Radiation Res. 2005;163:337–342. doi: 10.1667/rr3321. [DOI] [PubMed] [Google Scholar]
- 38.Miller JH, Zheng F. Large-scale simulations of cellular signaling processes. Parallel Comput. 2004;30:1137–1149. [Google Scholar]
- 39.Zheng F, Zhan CG. Modeling of pharmacokinetics of cocaine in human reveals the feasibility for development of enzyme therapies for drugs of abuse. PLoS Comput Biol. 2012;8:e1002610. doi: 10.1371/journal.pcbi.1002610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun H, Shen ML, Pang YP, Lockridge O, Brimijoin S. Cocaine Metabolism Accelerated by a Re-Engineered Human Butyrylcholinesterase. J Pharmacol Exp Ther. 2002;302:710–716. doi: 10.1124/jpet.302.2.710. [DOI] [PubMed] [Google Scholar]
- 41.Polhuijs M, Langenberg JP, Benschop HP. New Method for Retrospective Detection of Exposure to Organophosphorus Anticholinesterases: Application to Alleged Sarin Victims of Japanese Terrorists. Toxicol Appl Pharmacol. 1997;146:156–161. doi: 10.1006/taap.1997.8243. [DOI] [PubMed] [Google Scholar]
- 42.Bartels CF, Xie W, Miller-Lindholm AK, Schopfer LM, Lockridge O. Determination of the DNA Sequences of Acetylcholinesterase and Butyrylcholinesterase from Cat and Demonstration of the Existence of Both in Cat Plasma. Biochem Pharmacol. 2000;60:479–487. doi: 10.1016/s0006-2952(00)00365-8. [DOI] [PubMed] [Google Scholar]
- 43.Ge X, Zhang W, Lin Y, Du D. Magnetic Fe3O4@TiO2 nanoparticles-based teststripimmunosensing device forrapiddetectionofphosphorylatedbutyrylcholinesterase. Biosensors Bioelectron. 2013;50:486–491. doi: 10.1016/j.bios.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 44.Lockridge O, La Du BN. Comparison of Atypical and Usual Human Serum Cholinesterase. J Biol Chem. 1878;253:361–366. [PubMed] [Google Scholar]
- 45.Ralston JS, Main AR, Kilpatrick BF, Chasson AL. Use of procainamide gels in the purification of human and horse serum cholinesterases. Biochem J. 1983;211:243–250. doi: 10.1042/bj2110243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boyer CS, Pertersen DR. Enzymatic basis for the transesterification of cocaine in the presence of ethanol: Evidence for the participation of microsomal carboxylesterases. J Pharmacol Exp Therap. 1992;260:939–946. [PubMed] [Google Scholar]
- 47.Song N, Parker RB, Leizure SC. Cocaethylene formation in rat, dog, and human hepatic microsomes. Life Sci. 1999;64:2101–2108. doi: 10.1016/s0024-3205(99)00159-9. [DOI] [PubMed] [Google Scholar]
- 48.Yang J, Tucker GT, Rostami-Hodjegan A. Cytochrome P450 3A expression and activity in the human small intestine. Clin Pharmacol Ther. 2004;76:391. doi: 10.1016/j.clpt.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 49.Geng L, Gao Y, Chen X, Hou S, Zhan CG, Radic Z, Parks R, Russell SJ, Pham L, Brimijoin S. Gene transfer of mutant mouse cholinesterase provides high lifetime expression and reduced cocaine responses with no evident toxicity. PLoS One. 2013;8:e67446. doi: 10.1371/journal.pone.0067446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ladona MG, Gonzalez ML, Rane A, Raymund MP, de laTorre R. Cocaine metabolism in human fetal and adult liver microsomes is related to cytochrome P450 3A expression. Life Sci. 2000;68:431–443. doi: 10.1016/s0024-3205(00)00952-8. [DOI] [PubMed] [Google Scholar]