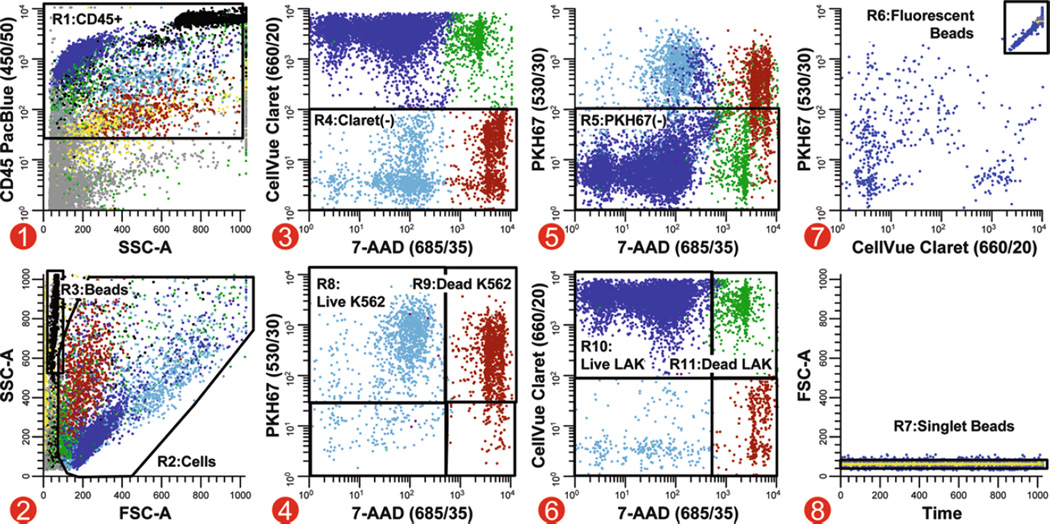
Fig. 5.
Analysis strategy for simultaneous quantitation of target and effector viability in a direct cytotoxicity assay. In this multicolor cytotoxicity assay, LAK effector cells stained with CellVue Claret (final concentrations: 5 × 107 cells/mL, 5 µM dye) and K562 targets stained with PKH67 (final concentrations: 1 × 107 cells/mL, 1 µM dye) were co-cultured at 37°C to assess LAK cell-mediated killing. Killing was assessed using two different metrics: (1) as % of targets able to exclude a viability probe (7-AAD) and (2) using counting beads to enumerate the number of viable target cells that remained when effectors were present versus when they were absent. Because these beads have very low forward scatter (R3, plot 2), it was not possible to set an acquisition threshold on this parameter and reliably ensure that all bead events were collected. Side scatter was, therefore, used as the thresholding parameter, with anti-CD45 PacBlue being used to include all leukocytes and beads (plot 1; see Note 41). A reciprocal gating strategy was applied to assess target and effector cell numbers and viability. A two-parameter plot of CellVue Claret versus 7-AAD fluorescence, gated on CD45+ events (R1, plot 1) with cell-like light scatter (R2, plot 2) that were not beads (not R6, plot 7), was used to identify target cells as events that were not CellVue Claret positive (R4, plot 3). Live versus dead target cells were then enumerated on a two-parameter plot of PKH67 versus 7-AAD fluorescence (plot 4, gated on R1&R2&R4 and not R6). Similarly, CellVue Claret-stained LAK cells were identified as PKH67 negative (R5) on a plot of CellVue Claret versus 7-AAD fluorescence (plot 5, gated on R1&R2 and not R6. This strategy was used because substantial differences in autofluorescence between targets and effectors (see Table 2) made it difficult to establish instrument settings that gave complete resolution between PKH67+ K562 targets and PKH67 negative (CellVue Claret+) LAK cells (plot 5), whereas much better resolution was possible between CellVue Claret + LAK cells and CellVue Claret negative (PKH67+) K562 cells (plot 3). Counting beads (R6), which exhibit broad-spectrum fluorescence (plot 7, gated on R3), were enumerated in plot 8 (gated on R3&R6) after gating on R7 to exclude doublets and larger aggregates. Extent of cytotoxic killing was assessed as described in Subheading 3.3.4, steps 5 and 6. Color codes for plots 1–6: blue = viable LAK effectors; green = nonviable LAK effectors; light blue = viable K562 targets; red-brown = dead K562 targets; black = enumeration beads; gray = noise/debris; yellow = very low forward scatter events.