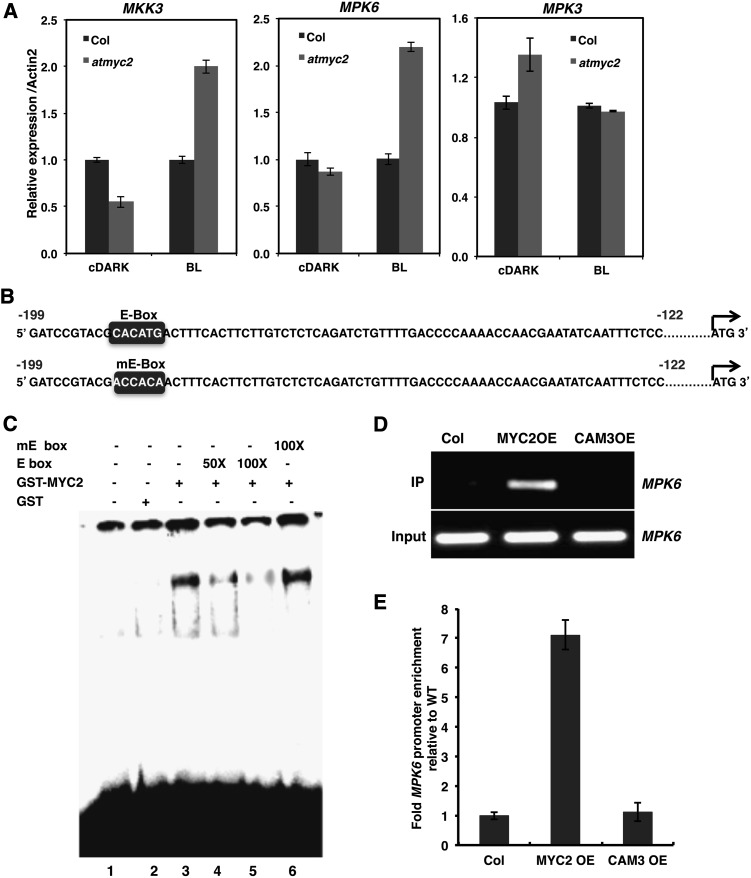
Figure 1.
MYC2 Directly Binds to the E-Box of MPK6 Promoter.
(A) Real-time PCR analysis of MKK3, MPK6, and MPK3 transcripts in 6-d-old constant dark-grown seedlings transferred to BL (30 μmol m−2 s−1) for 10 min. ACT2, internal control. Error bars indicate sd. n ≥ 3 independent experiments with similar results.
(B) Wild-type (E-box) and mutated (mE-box) E-box containing MPK6 promoter fragments used for the gel shift assays.
(C) Electrophoretic mobility shift (gel shift) assays showing that MYC2 specifically binds to the E box (−181 to −186 bp upstream of ATG). Approximately 500 ng of recombinant protein was added (lanes 3 to 6) to the radioactively labeled 78-bp MPK6 promoter fragment containing the E-box. No protein was added in lane 1, and 500 ng of GST protein was added in lane 2. The 50 and 100 molar excess of wild type E-box containing promoter fragment was added in lanes 4 and 5, and 100 molar excess of mutated E-box containing promoter fragment was added in lane 6 as competitors. The plus and minus signs indicate the presence or absence of competitors, GST-MYC2, or GST.
(D) ChIP assays of the MPK6 promoter from Col, MYC2 overexpresser (MYC2-cMycOE), and Cam3OE transgenic seedlings grown in constant BL (30 μmol m−2 s−1) for 10 d (using antibodies to cMyc). The gel image shows the results of PCR amplification of MPK6 promoter fragment in the immunoprecipitate and input.
(E) Results of real-time qPCR are presented as fold enrichment relative to the wild type (Col). ChIP values were first normalized by the respective input values and then fold enrichment relative to the wild type was calculated. Error bars represent se (n = 3).