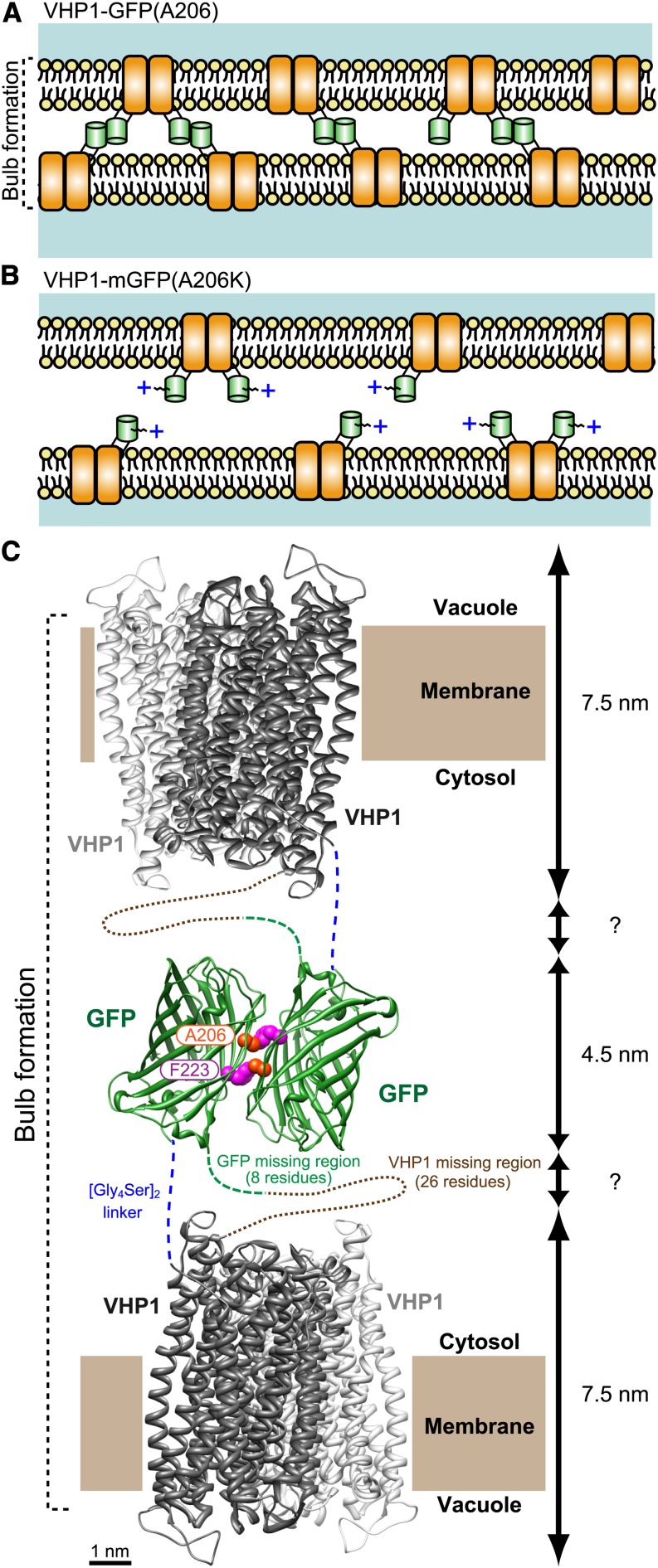
Figure 7.
Schematic Model of Tonoplast Adhesion via VHP1-GFP Dimerization.
(A) In VHP1-GFP-expressing cells, VHP1-GFPs form dimers with molecules in the opposite-side membrane and generate bulb structures. In the GFP homodimer, the Ala-206 residue interacts with Phe-223 of its partner GFP by a hydrophobic bond (Yang et al., 1996).
(B) In VHP1-mGFP-expressing cells, the positive charge and steric hindrance of the Lys-206 side chain completely block GFP dimerization. As a result, adjacent tonoplasts are kept at a proper distance from each other.
(C) Schematic model of tonoplast adhesion via VHP1-GFP dimerization. H+-PPase exists as a dimer as shown by Lin et al. (2012) (PDB ID: 4A01; Vigna radiata VHP1). The dimeric structure of GFP is taken from PDB ID: 1GFL (Yang et al., 1996). Modeling of the molecular structure of the dimeric form of VHP1-GFP was made with UCSF Chimera software (a visualization system for exploratory research and analysis; www.cgl.ucsf.edu/chimera/download.html). Ala-206 of GFP interacts with Phe-223 in another GFP by a hydrophobic bond, and a homotypic dimer is formed. Total thickness of the double tonoplast is estimated to be around 20 nm when the region of the [Gly4Ser2] stacked linker is a few nanometers. This value is comparable to the thickness shown in Supplemental Figure 5J.