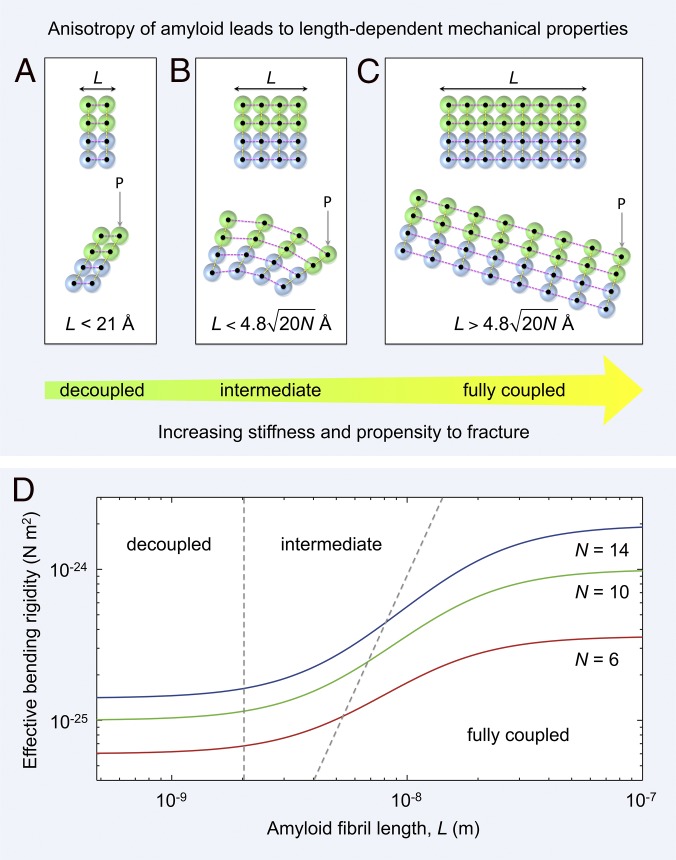
Fig. 5.
Mechanical anisotropy of amyloid leads to length-dependent material properties. (A–C) An amyloid fibril is a network of rigid β-strands (colored spheres) interconnected via elastic (strong) longitudinal (magenta dashed lines) and (weak) lateral bonds (yellow dashed lines). Amyloid fibrils of different lengths, L, along the hydrogen-bonding axis are shown schematically as two laterally connected protofilaments (green and blue spheres represent the first and second protofilament, respectively). (A) A short fibril (Upper) bends under a load P through shearing of lateral intersheet and interprotofilament bonds [Lower, decoupled regime (36)]. (B) Fibrils of intermediate length (Upper) bend through a combination of extension or compression of longitudinal bonds and shearing of lateral intersheet and interprotofilament bonds [Lower, intermediate regime (36)]. (C) For long fibrils (Upper), longitudinal bonds stretch or compress during bending, with shear contributions becoming negligible [Lower, fully coupled regime (36)]. (D) The predicted shear-weakening effect (36) on the effective bending rigidity of fibrils is plotted as a function of fibril length (SI Methods). Data are plotted for doublet (red line), triplet (green line), and quadruplet (blue line) fibril polymorphs formed by TTR(105-115) (4). The boundaries between decoupled, intermediate, and fully coupled bending are shown as gray dashed lines.