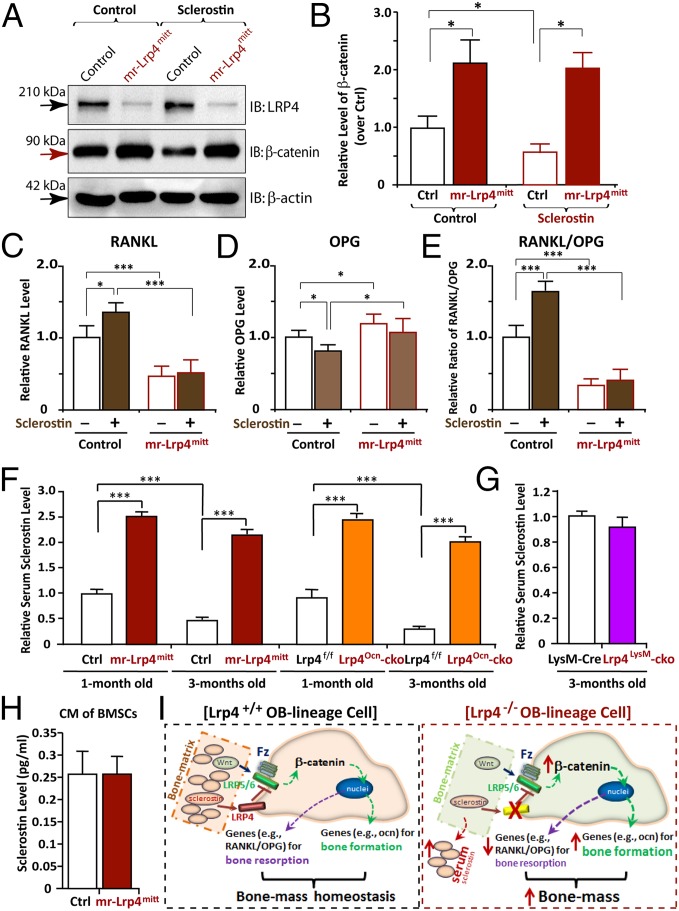
Fig. 6.
Impairment of sclerostin reduction of β-catenin and induction of RANKL/OPG in Lrp4-deficient BMSCs and increase of serum sclerostin in both mr-Lrp4mitt and Lrp4Ocn-cko, but not Lrp4LysM-cko, mice. (A and B) Increase of β-catenin level and impairment of sclerostin reduction of β-catenin in Lrp4-deficient BMSCs. BMSCs were treated with control CM or sclerostin CM for 12 h. Cell lysates were subjected to Western blot analyses using indicated antibodies. Quantification analysis by ImageJ software is shown in B. The values of mean ± SD from three different experiments were presented. Data were normalized by β-actin loading and WT controls. *P < 0.05, significant difference. (C–E) Failure of sclerostin induction of RANKL/OPG in Lrp4-deficient BMSCs. BMSCs were treated with control CM or sclerostin CM for 24 h. The mRNAs were subjected to real-time PCR analyses. The values are normalized to β-actin and WT controls. Means ± SD values from three different experiments are presented. *P < 0.05; ***P < 0.01, significant difference. (F and G) Increased serum levels of sclerostin in both mr-Lrp4mitt and Lrp4Ocn-cko, but not Lrp4LysM-cko, mice. (H) A comparable level of CM–sclerostin between WT control and Lrp4 mutant BMSCs. In F–H, the serum and CM–sclerostin were measured by ELISA analysis (SI Materials and Methods). Means ± SD values from three different animals per genotype or three different cultures are presented. Data were normalized to their controls. Note that the control values of serum sclerostin in HSA–Lrp4 mice (1 mo old), Lrp4f/f (1 mo old), and LysM–Cre (3 mo old) were 750, 700, and 350 pg/mL, respectively. ***P < 0.01, significant difference. (I) Illustration of a working model for Lrp4 in OB-lineage cells to serve as a receptor of sclerostin, sequestering sclerostin into bone matrix, suppressing Wnt/β-catenin signaling and bone formation, and promoting RANKL-induced OC genesis and bone resorption, thus maintaining bone-mass homeostasis.