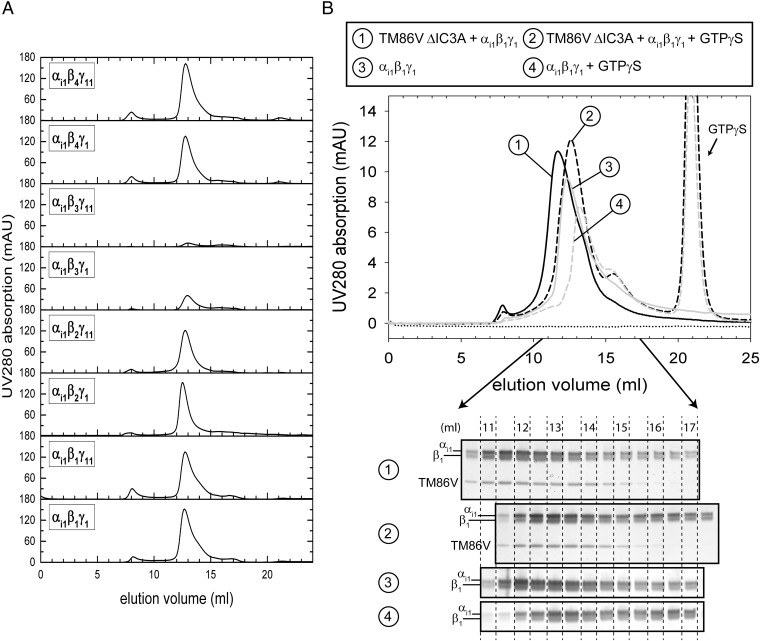
Fig. 5.
Purification of heterotrimeric G-protein complexes and GPCR/G-protein complex formation. (A) Final size-exclusion profiles of the preparative purification of eight G-protein combinations that performed best in interaction with NTR1. Runs were performed on a Superdex 200 10/300 GL column equilibrated in size-exclusion buffer [10 mM Hepes, pH 8.0, 200 mM NaCl, 1 mM MgCl2, 10 µM GDP, 2 mM DTT, 0.3% (wt/vol) DM]. (B) Analytical size-exclusion profiles after dialysis of TM86V ΔIC3A/αi1β1γ1 complex (black lines, #1 and 2) or αi1β1γ1 alone (gray lines, #3 and 4) in the absence (solid lines, #1 and 3) or presence (dashed lines, #2 and 4) of 100 µM GTPγS. GTPγS alone has an elution volume of 21 mL. TM86V ΔIC3A alone (dotted line) precipitated during dialysis. Proteins were dialyzed against dialysis buffer [20 mM Hepes, pH 7.4, 100 mM NaCl, 2 mM DTT, 3 mM MgCl2, and 0.02% (wt/vol) DDM], and the Superdex 200 10/300 GL column was equilibrated in the very same buffer. Fractions of 0.5 mL were collected, and protein-containing fractions were analyzed by silver-stained gels. Shown are the bands corresponding to αi1, β1, and TM86V ΔIC3A.