Figure 1.
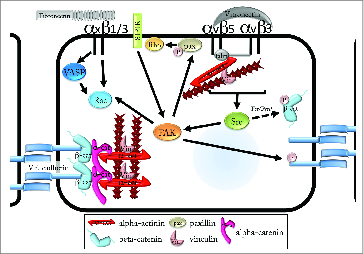
Intracellular regulation of VE-cadherin by integrins. Activation of αvβ5 or αvβ3 causes activation of c-Src (Src) and subsequent activation of focal adhesion kinase (FAK). FAK phosphorylates tyrosine 658 on VE-cadherin, prompting loss of cadherin adhesion. FAK also phosphorylates paxillin (pax), which leads to the activation of Rho GTPase and the formation of radial stress fibers. Alternatively, the Src-family kinases Fer and Fyn phosphorylate tyrosines 654 and 142 on β−catenin. While Fer and Fyn can be activated downstream of integrin ligation, no evidence yet exists for integrin-mediated phosphorylation of β−catenin. Activation of β1 and β3 integrins conversely promotes cadherin adhesion. Activation of FAK downstream of sphingosine-1-phosphate recptor-1 (S1P1R) as well as integrin-dependent activation of vasodilator-stimulated phosphoprotein (VASP) triggers activation of Rac1 GTPase. Rac then promotes the stability of cortical actin and of cadherin adhesions.
