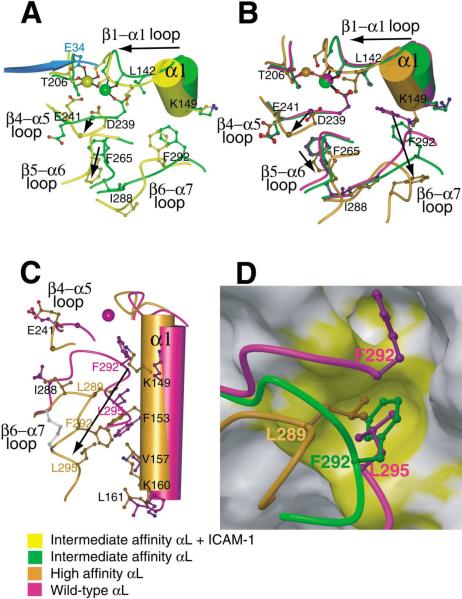
Figure 4. Propagation of Conformational Change in the I Domain.
(A–C) The effect of ligand binding (A) and remodeling of the β6-α7 loop in absence of ligand binding (B and C) are compared on I domain structures near the MIDAS (A and B) and the α7-α1 interface (C). Views of (A) and (B) are similar and (C) is approximately orthogonal to (B). Key residues in conformational change are shown in ball-and-stick and labeled, except Gly-240 is unlabeled and shown as a large Cα atom sphere and MIDAS residues Ser-139 and Ser-141 are shown but not labeled. Metal ions are shown as large spheres with the same color as the backbone of the corresponding I domain. The position of the missing metal ion in the unliganded high-affinity structure is simulated by superimposing the metal ion from the pseudo-liganded high-affinity structure and is shown smaller than the other metals. Side chain oxygen atoms are shown as red; water oxygens are omitted for clarity. The directions of major shifts from closed to open conformations are shown with arrows. A portion of ICAM-1 domain-1 containing the metal-coordinating residue E34 is shown in cyan. In (C), the side chain bonds in the designed disulfide bridge (Cys-287-Cys-294) are shown in gray.
(D) The hydrophobic pocket that acts as a detent for the rachet-like movement of the β6-α7 loop. The backbone of the β6-α7 loop and the three residues that occupy the same hydrophobic pocket in the three different conformational states are shown in the same color key as in (A)–(C). The pocket is shown as a GRASP van der Waals surface using the wild-type 1LFA structure with the residues from 287 to the C terminus deleted. The upper hydrophobic pocket is also shown, which is occupied only in the closed conformation (by F292 which is shown in the wild-type structure along with L295). For the closed conformation, the β6-α7 main chain trace is broken between F292 and L295 for clarity. The view is about the same as in (C). On the otherwise gray GRASP surface, residues Ile-150, Phe-153, Ile-237, Ile-259, and Ile-261 are colored yellow to show the hydrophobic pockets.