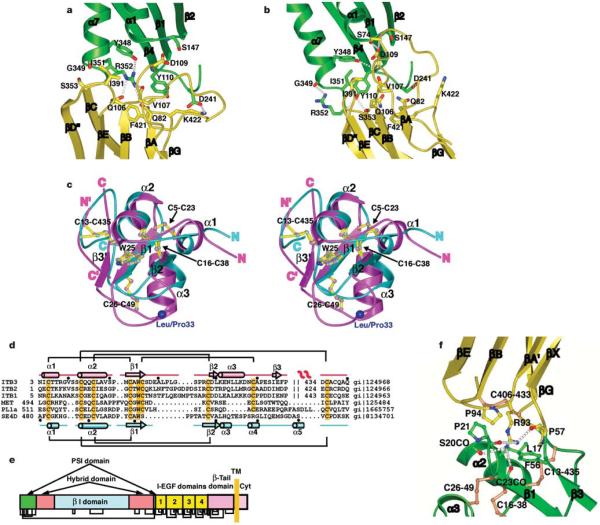
Figure 5.
The hybrid and PSI domains and their interfaces. a, b, The β I/hybrid domain interface in the unliganded-closed structure7 (a) and liganded-open structure (b). Ribbon backbone and side-chain carbon atoms are shown in green (β I) and yellow (hybrid) with theb I domainb β-sheet in the same orientation. The α7-helical ribbon is shown up to the same residue (350) in both structures to aid comparison of α7-helix position. c, Stereo view of the superposition of the PSI domains fromβ3 (magenta) and semaphorin4D (cyan). The disulphide bridges and the conserved tryptophan are shown as ball-and-stick models with their bonds coloured yellow and atoms the same colour as the backbone. The Leu/Pro 33 alloantigen site is represented with a large blue Ca sphere. The amino and carboxyl termini of each domain are indicated. The N’ and C’ refer to termini for residues 434–440 that constitute part of the PSI and EGF-1 domains. d, Sequence alignment of PSI domains from integrins, semaphorin4D (SE4D), a plexin and c-met. Disulphide connections are shown above (β3) and below (semaphorin4D) the respective sequences. The conserved cysteines and tryptophans are highlighted orange. The secondary structures are shown for β3 (top) and sema4D (bottom). e, Domain organization of integrin β subunits, showing multiple domain insertions. The revised disulphide bond pattern is shown below. f, The interface between the hybrid (yellow) and PSI (green) domains. Disulphide bonds are shown in orange.