Figure 3.
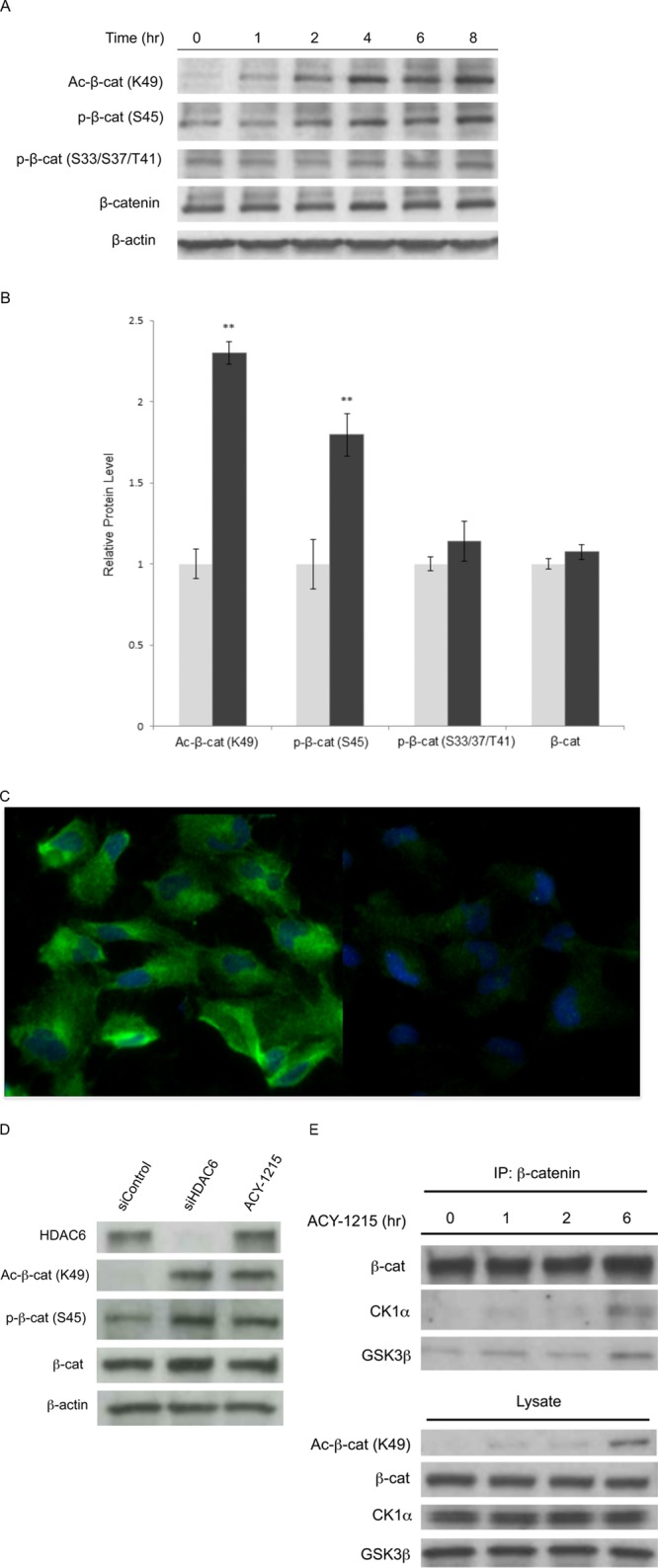
Effect of HDAC6 inhibitor-mediated increase in acetylation of β-catenin Lys49 on N-terminal posttranslational modification sites. (A) Time-course immunoblot analysis of human NPCs treated with an HDAC6 inhibitor. Human NPCs were treated with HDAC6 inhibitor ACY-1215 at 5 μM for the times points indicated and the lysates immunoblotted with antibodies against β-catenin, Ac-Lys49-β-catenin, phos-Ser45 and phos-Ser33, Ser37, and Thr41. β-actin is using shown as loading control. (B) Quantification of the intensity of Western blot band signals from A. in indicated antigens normalized to total β-catenin before and after treatment with ACY-1215 at 5 μM for 8 h. Results are representative of three independent experiments. Error bars indicate standard deviation. ** and *** denote significance with p < 0.01 and p < 0.001, respectively (paired t test). (C) Immunofluorescence staining for HDAC6 in human NPCs when treated with control siRNA (left) and HDAC6 siRNA (right). (D) Immunoblot analysis of lysates from human NPCs treated with HDAC6 siRNA, control siRNA, and ACY-1215, using antibodies against HDAC6, β-catenin, Ac-Lys49-β-catenin, and phos-Ser45-β-catenin. β-actin is shown as loading control. (E) Time course of CK1α and GSK3β bound to β-catenin bound to in human NPCs treated with 5 μM ACY-1215. Cell lysates were immunoprecipitated with anti-β-catenin antibody and levels of CK1α and GSK3β were measured by immunoblot analysis.
