Figure 2.
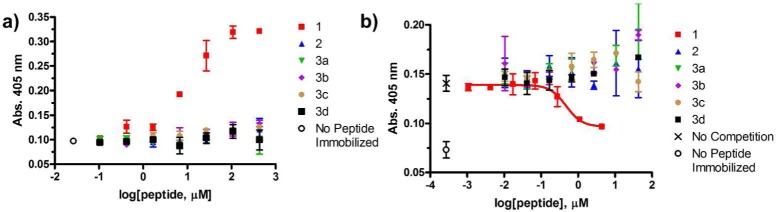
Commercial polyclonal anti-BimBH3 antibody preparation binds to an α-peptide derived from BimBH3 (1) but not to α/β homologues (2,3a-d). Data points represent mean ± SD, with each condition run in duplicate. (a) Indirect ELISA analysis of anti-BimBH3 commercial antibody binding to immobilized synthetic BimBH3 analogues. The x-axis represents the concentration of peptide in solutions used during the plate-coating step. “No peptide immobilized” is a negative control. (b) Competitive ELISA analysis of peptides in solution inhibiting the binding of commercial anti-BimBH3 antibody to immobilized peptide 1. The x-axis represents the concentration of peptide incubated with polyclonal antibody before adding this solution to wells containing immobilized α-peptide 1. “No competition” denotes the absorbance observed when no competitor peptide was incubated with polyclonal antibody prior to addition. The red curve represents the fitting of a sigmoidal dose response model to the data for α-peptide 1 (IC50 = 3 μM). None of the α/β-peptide analogues inhibits binding to immobilized 1 at the highest α/β-peptide concentration tested (43 μM).
