Figure 3.
Specific Loss of Inhibitory Interneurons after AAV.flex.DTA Injection into the Dorsal Horn of GlyT2::Cre+ Mice
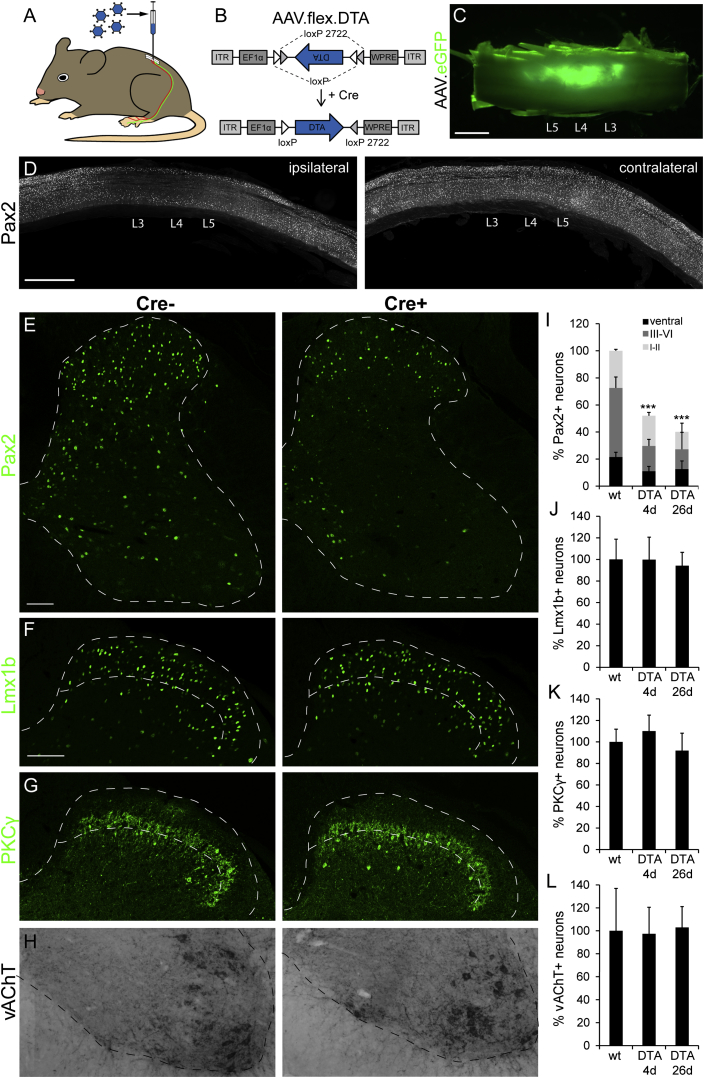
(A) AAV.flex.DTA was injected into the lumbar dorsal horn of GlyT2::Cre+ GlyT2::Cre− littermates.
(B) The AAV.flex.DTA genome. The expression of DTA is driven by the EF1a promotor (EF1a) and depends on Cre-mediated irreversible inversion of the DTA coding sequence.
(C) Green fluorescence illustrates virus spread (of an AAV.eGFP) following three separate unilateral injections into the lumbar spinal cord at levels L3–L5.
(D) Sagittal sections of a spinal cord after injection of AAV.flex.DTA illustrate a marked reduction in the number of Pax2+ cells on the ipsilateral side (left) but not on the contralateral side (right).
(E) Compared with AAV.flex.DTA-injected GlyT2::Cre− control mice, a clear loss of Pax2+ cells was observed 4 days after injection of AAV.flex.DTA on the injected side of GlyT2::Cre+ mice. Fluorescence signals detected by confocal microscopy were false-colored in green.
(F–H) Same as (E) but analysis of Lmx1b+ excitatory dorsal horn neurons (F), PKCγ+ excitatory dorsal horn neurons (G), and vAChT+ motoneurons (H).
(I–L) Quantification (mean ± SD). At least three to four horizontal sections per mouse centered around the L4 injection site were used for quantification.
(I) ∗∗∗p < 0.001 versus baseline, one-way ANOVA, F(2,33) = 59.1, followed by Bonferroni post hoc test.
(I–K) No significant differences in cell counts were found for Lmx1b+, PKCγ+, or vAChT+ neurons (p > 0.20). Dashed lines indicate gray matter border (E–H) and borders of lamina II (F and G).
Scale bars, 1 mm (C and D) and 100 μm (E and H).