Figure 4. Pharmacokinetic modeling of 18F-VUIIS1008.
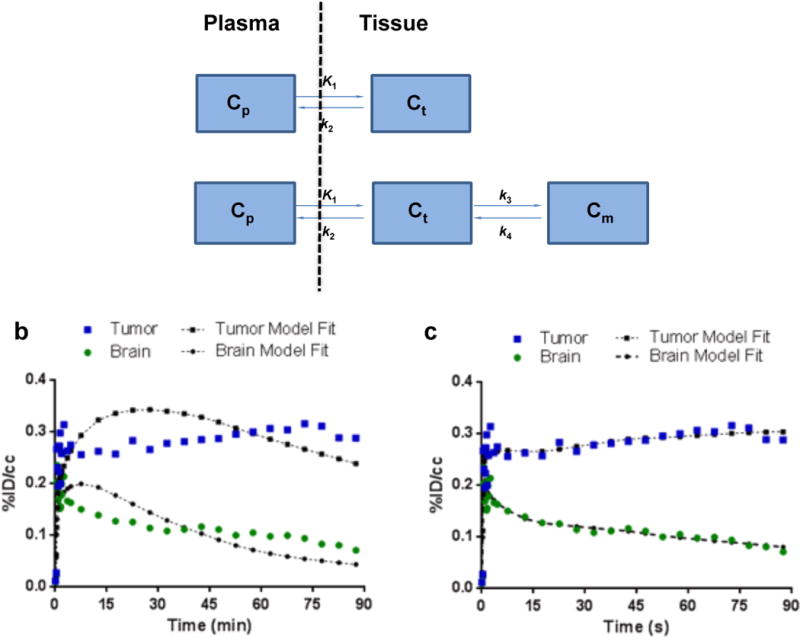
(a) Pharmacokinetic models evaluated to describe 18F-VUIIS1008 kinetics in tumor and brain. In these two models: Cp represents the concentration of 18F-VUIIS1008 in the plasma; Ct represents the concentration of tracer in a nonspecific compartment; Cm represents the concentration of tracer in a specific binding compartment. The parameters of K1, k2, k3, and k4 reflect transport between the compartments. (b) Representative TACs and pharmacokinetic model fits for 18F-VUIIS1008 in a 1-tissue, 2-kinetic-parameter model (calculated χ2 for one-tissue modeling fit = 26.311 ± 5.210; Mean ± SEM, n = 5). (c) Representative TACs and pharmacokinetic model fits for 18F-VUIIS1008 in a 2-tissue, 4-kinetic-parameter model (calculated χ2 for two-tissue modeling fit = 9.458 ± 2.623; Mean ± SEM, n = 5). TACs for tumor (blue) and contralateral brain (green) are shown with associated model fit. %ID/cc = percentage injected dose per cubic centimeter.
