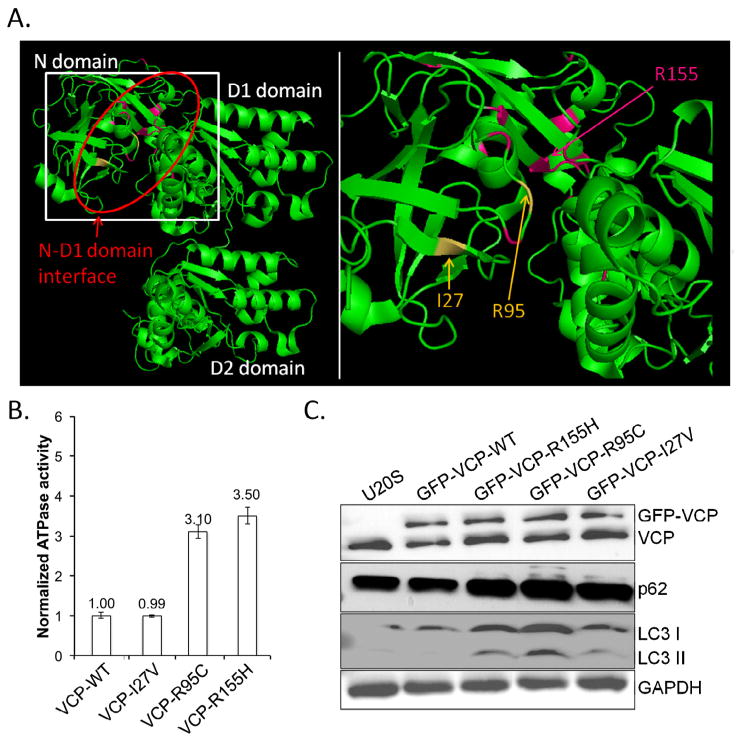
Fig. 1.
A) Crystallographic rendering of a single VCP monomer with the N, D1 and D2 domains indicated. Red oval emphasizes the N-D1 domain interface where all known disease mutations reside. Disease mutated residues are in pink (R155 is the most commonly mutated residue) and the I27 and R95 residues mutated in this report are in yellow. Box denotes region of enlargement. B) Recombinant human wild-type VCP (VCP-WT), or disease mutant associated VCPs (VCP-I27V, VCP-R95C and VCP-R155H) were purified from E. coli and the basal ATP hydrolysis rate assayed. The ATPase activity of VCP-WT was arbitrarily designated as 1. C) Representative immunoblot from three independent experiments for VCP, LC3, p62/SQSTM1 and actin of U20S cells transiently expressing VCP-WT-GFP, VCP-R155H-GFP, VCP-R95C-GFP or VCP-I27V-GFP for 2 days. Note the selective increase in both p62/SQSTM1 and LC3II in disease mutant expressing cells.