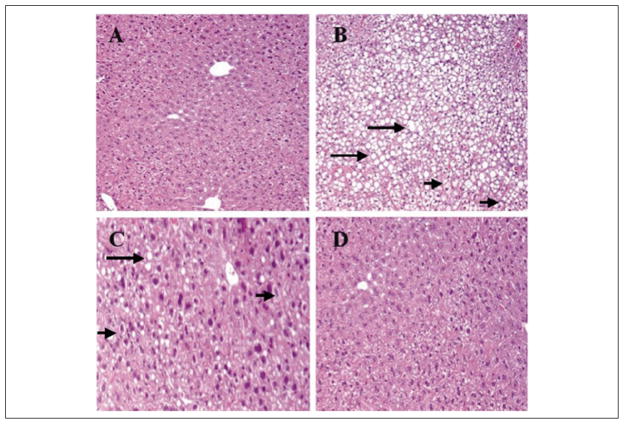
Figure 2.
Representative hematoxylin and eosin–stained sections from liver tissue of mice. (A) Control group shows normal hepatocytes with absence of either steatosis or ballooning degeneration. (B) The PN-O group demonstrates severe steatosis (grade 3), with ballooning degeneration (grade 2). (C) The PN–ω-6 group shows mild steatosis (grade 1) with ballooning degeneration of some hepatocytes. (D) The PN–ω-3 group shows normal hepatocytes with absence of either steatosis or ballooning degeneration. In these sections, examples of steatosis are indicated by the long arrows and ballooning degeneration by the short arrows (A, B, D = ×10 magnification; C = ×20 magnification). PN-O, oral, fat-free parenteral nutrition (PN) solution only; PN–ω-6, PN-O plus intraperitoneal (IP) ω-6 fatty acid (FA)–predominant supplements; PN–ω-3, PN-O plus IP ω-3 FA.