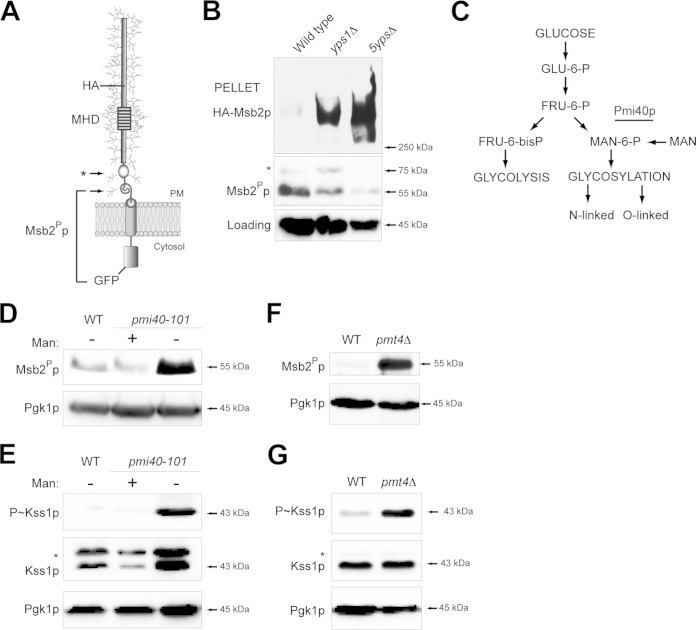
FIG 1.
Underglycosylated Msb2p is proteolytically processed at elevated levels. (A) The Msb2p protein is shown as a single-pass glycoprotein with the mucin homology domain (MHD). Cleavage sites corresponding to immunoblot data for cleaved Msb2p-GFP are indicated by arrows. Msb2Pp refers to the proteolytically processed form; the asterisk refers to a minor cleavage product. The positions of HA and GFP fusions are shown. (B) Cleavage of HA-Msb2p-GFP in the yps1Δ and 5ypsΔ mutants. The top blot was probed with anti-HA antibodies to show full-length Msb2p at >250 kDa (HA-Msb2p). The middle blot was probed with anti-GFP antibodies to show the proteolytically processed Msb2Pp-GFP fusion (Msb2Pp, 55 kDa; *, 75 kDa). Blots were probed with anti-Pgk1p antibody, which was used as a loading control for all experiments. (C) Pathway for the conversion of glucose into substrates for glycolysis and protein glycosylation. The Pmi40p enzyme is underlined. (D) Immunoblot of Msb2Pp in wild-type cells (WT) and the pmi40-101 mutant grown in YEPD (−Man) or YEPD plus 50 mM mannose (+Man) for 5.5 h. (E) Immunoblot of P∼Kss1p levels for the strains used in panel D. The asterisk refers to a background band seen under some conditions with the total Kss1p antibodies. (F) Msb2Pp levels in wild-type (WT) cells and the pmt4Δ mutant. Cells were grown in YEP-GAL for 6 h. (G) P∼Kss1p levels for the strains examined in panel F.