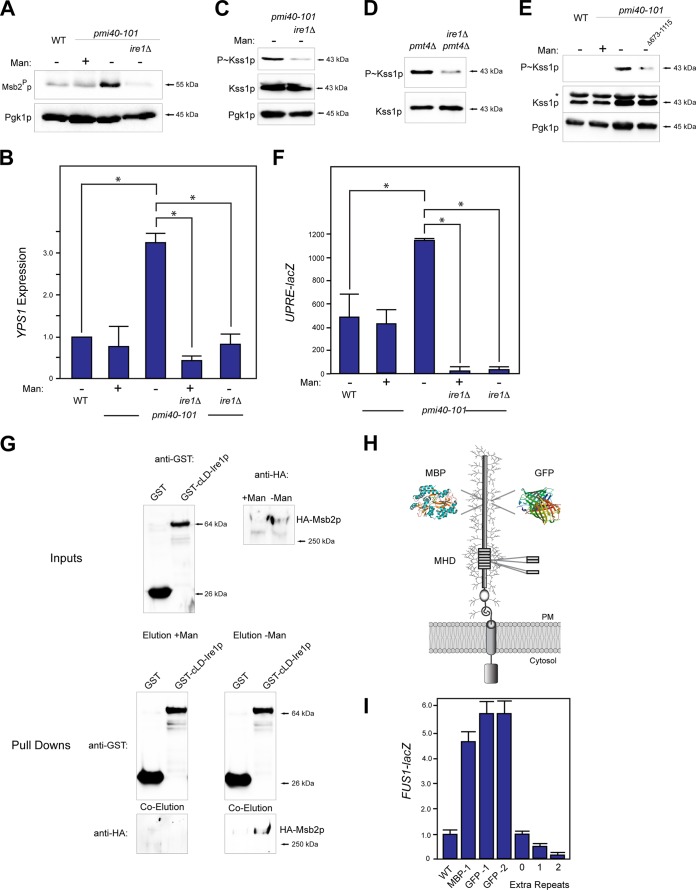
FIG 2.
Ire1p regulates Msb2p cleavage and filamentous-growth pathway activity by regulating YPS1 expression in response to protein glycosylation deficiency. (A) Msb2p cleavage in wild-type cells and the pmi40-101 mutant grown with (YEPD + Man) or without (YEPD) mannose in combination with the ire1Δ mutant. (B) YPS1 expression was determined by qPCR and adjusted to ACT1 levels as a control. The indicated strains were grown in YEPD (−Man) or YEPD plus Man medium (+Man) for RNA preparation and qPCR analysis. The asterisk refers to a P value of <0.05. (C) P∼Kss1p levels in the pmi40-101 and pmi40-101 ire1Δ double mutant. (D) P∼Kss1p levels in the pmt4Δ and pmt4Δ ire1Δ double mutants grown in YEP-GAL. (E) P∼Kss1p levels for an Ire1p C-terminal truncation. (F) UPRE-lacZ activity was determined by β-galactosidase assays for the indicated strains and conditions. Experiments were performed in duplicate, and the average values are shown. Error bars represent the standard deviations between trials. The asterisk refers to a P value of <0.05. (G) In vitro pulldown of HA-Msb2p expressed in the pmi40-101 mutant in YEPD (with or without mannose) with the luminal domain of Ire1p, called cLD-Ire1p-GST. Input, pulldown, and coelutions are shown. (H) Msb2p with insertion of tandem repeats, MBP, or GFP shown. GFP-1 was inserted at residue 324, resulting in an in-frame deletion of aa 324 to 326. GFP-2 was inserted at residue 246 and resulted in a deletion of aa 246 to 539. MPB was inserted at residue 324 without deletion of amino acid residues. (I) MAPK activity was assessed by an Msb2p-dependent reporter (FUS1-lacZ, which in Σ1278b ste4 strains shows Msb2p dependence [15]). Differences are expressed as fold differences compared to those of wild-type cells. Error bars represent standard deviations between trials, which varied by less than 10%.