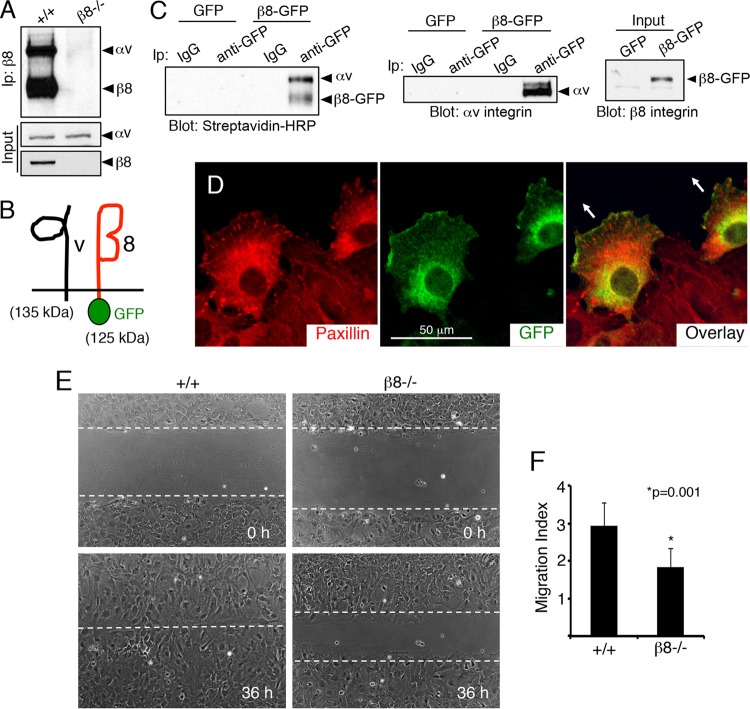
FIG 1.
β8 integrin promotes directional cell migration in vitro. (A) αvβ8 integrin is expressed in primary astrocytes. Biotinylation and immunoprecipitation (Ip) with an anti-β8 integrin antibody revealed cell surface integrin protein expression in wild-type (+/+) cells and a complete absence of αvβ8 integrin dimers in β8−/− cells. Inputs show that αv integrin is expressed in the absence of β8 gene expression. (B) Schematic showing an engineered 125-kDa protein comprised of GFP fused to the C terminus of mouse β8 integrin which dimerizes with the 135-kDa αv integrin subunit. (C) Astrocytes expressing GFP or β8-GFP were cell surface biotinylated and immunoprecipitated with control IgG antibodies or anti-GFP antibodies and then labeled with streptavidin-HRP. Note that β8-GFP forms cell surface complexes with αv integrin (left). Astrocytes expressing GFP or β8-GFP were lysed and immunoprecipitated with anti-GFP and immunoblotted with anti-αv integrin (right). (D) Confluent monolayers of astrocytes infected with lentiviruses expressing β8-GFP protein were scratched and then immunolabeled with antipaxillin (left) and anti-GFP (middle), revealing that β8-GFP is enriched at the leading edge of migrating cells (overlay, right). Arrows in the right panel indicate the direction of migration. (E) Confluent monolayers of wild-type and β8−/− astrocytes were scratched, and directional cell migration was imaged over 36 h. (F) Quantitation of integrin-dependent migration defects in scratch-wound assays.