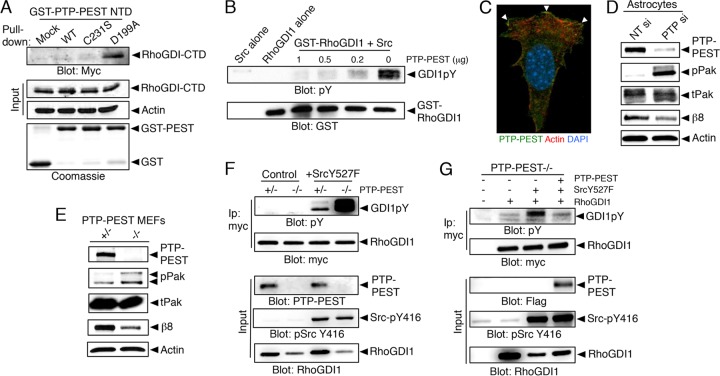
FIG 6.
Integrin-bound PTP-PEST dephosphorylates RhoGDI1. (A) Substrate trapping experiments with the wild-type PTP-PEST catalytic domain or catalytically inactive point mutants reveal binding between the hyperphosphorylated RhoGDI1 CTD and the D199A mutant construct. (B) The PTP-PEST catalytic domain promotes dephosphorylation of the Src-phosphorylated RhoGDI1 protein in vitro. The GST-RhoGDI1 protein was tyrosine phosphorylated in vitro by mixing with purified Src and ATP. GST-RhoGDI1 complexes were then incubated with various amounts of the PTP-PEST catalytic domain. Glutathione-agarose was used to fractionate GST-RhoGDI1, and proteins were immunoblotted with anti-GST or anti-pY antibodies. Controls were Src alone or GST-RhoGDI1 alone in the absence of PTP-PEST. (C) Mouse astrocytes transfected with a Flag-tagged PTP-PEST construct show PTP-PEST protein enrichment at the membrane. DAPI, 4′,6-diamidino-2-phenylindole. (D) Silencing of PTP-PEST gene expression in astrocytes using siRNAs leads to increased levels of phosphorylated Pak, indicating hyperactive Rac1/Cdc42 signaling. Also note the decrease in β8 integrin protein levels after PTP-PEST silencing. All immunoprecipitation and GST pulldown experiments were performed at least three different times. Nontargeting (NT) si and PTP si, siRNAs specific for NT and PTP. (E) MEFs genetically null for PTP-PEST show elevated levels of pPak proteins and express reduced levels of β8 integrin protein. (F) The levels of tyrosine-phosphorylated RhoGDI1 in control or PTP-PEST−/− MEFs expressing RhoGDI1-myc and constitutively active Src (Y527F mutant cells) were analyzed. Note that cells lacking PTP-PEST show elevated levels of tyrosine-phosphorylated RhoGDI1. (G) PTP-PEST−/− MEFs stably expressing constitutively active Src (Y527F mutant cells) show elevated levels of tyrosine-phosphorylated RhoGDI1 protein. These levels are reduced upon forced expression of PTP-PEST.