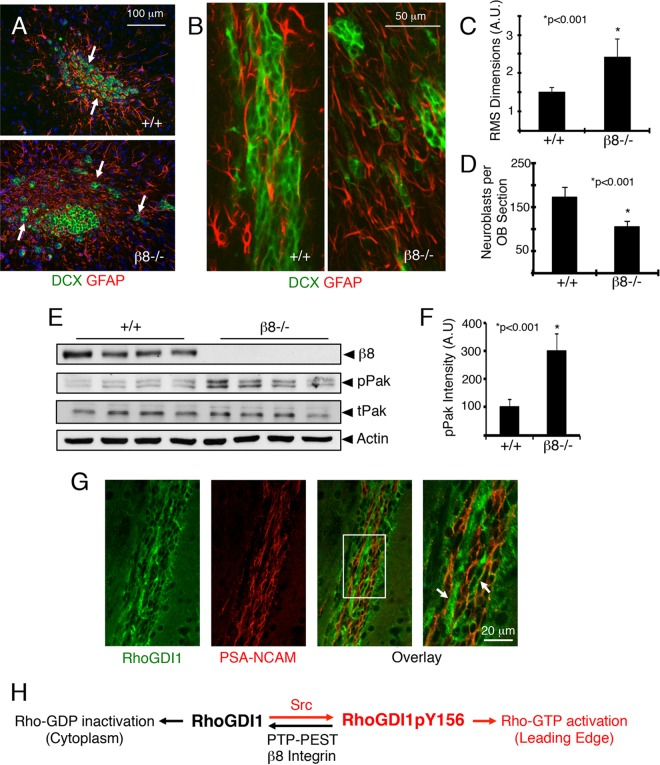
FIG 8.
β8 integrin promotes directional cell migration in vivo. (A) Representative coronal sections through the RMS of wild-type and β8−/− mice stained with anti-Dcx and anti-glial fibrillary acidic protein (anti-GFAP) reveal neuroblasts and astrocytes, respectively, in the RMS. Note the neural cell migration defects in β8−/− mice. (B) Representative sagittal sections through the RMS of wild-type and β8−/− mice labeled with anti-Dcx and anti-GFAP antibodies. Note the abnormal clusters of neuroblasts in β8−/− brain sections. (C, D) Quantitation of integrin-dependent neuroblast migration defects in the RMS (C) and olfactory bulbs (OBs) (D). (E) Tissue lysates were prepared from the SVZ/RMS of wild-type and β8−/− mice and immunoblotted with anti-phospho-Pak, a readout for Rac1/Cdc42 activation. tPak, total Pak; pPak, phosphorylated Pak. (F) Quantitation of integrin-dependent phospho-Pak protein levels. (G) RhoGDI1 protein is expressed in neuroblasts in the mouse rostral migratory stream, as revealed by colocalization with polysialylated neural cell adhesion molecule (PSA-NCAM). The right image is a higher-magnification image of the white boxed area. Arrows indicate neuroblasts expressing RhoGDI1 and PSA-NCAM. (H) In migrating cells, Src phosphorylation of RhoGDI1 on Y156 leads to deposition of Rac1/Cdc42 proteins at the leading edge, enabling their activation. RhoGDI1pY156 also translocates to the leading edge, where it is coupled to PTP-PEST via interactions with αvβ8 integrin, which facilitates RhoGDI1 dephosphorylation, release from the membrane, and subsequent suppression of Rac1/Cdc42 signaling in the cytoplasm. Collectively, these events control the spatiotemporal patterns of Rac1/Cdc42 activation to promote directional cell motility.