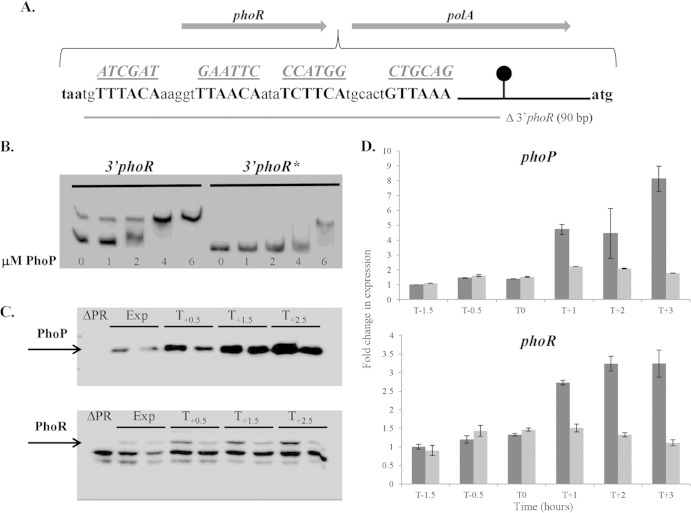
FIG 4.
Binding of PhoP∼P to the 3′ end of the phoPR operon is required for positive autoregulation. The role of PhoP∼P binding to the 3′ end of the phoPR operon was examined by gel shift (B), Western (C), and RT-qPCR (D) analyses. (A) The sequence of the PhoP boxes (boldface) positioned between the phoR stop codon (taa, in boldface) and the beginning of polA (atg, in boldface). The putative PhoP boxes are shown in boldface capital letters, while the sequences to which they are mutated are shown underlined above each motif. The region deleted in strain LSB046 is shown by a gray bar below the sequence. The phoPR transcriptional terminator is represented by a black line and circle. (B) An electrophoretic mobility shift assay was performed to verify PhoP∼P binding to DNA encoding the PhoP∼P boxes at the 3′ end of the phoPR operon. 3′phoR, a DNA fragment containing the wild-type PhoP boxes; 3′phoR*, a DNA fragment containing mutated (as shown in panel A) PhoP boxes. Each reaction mixture contained 2 ng of biotin-labeled DNA fragment, and the concentration of PhoP used in each reaction is indicated below the figure (0 to 6 μM). (C) Western blot analysis of PhoP and PhoR protein levels in strains 168 (wild-type) and LSB088 (mutated PhoP∼P boxes at the 3′ end of the phoPR operon). Both strains were grown in LPDM, and samples were harvested during exponential growth (Exp) and at 0.5 h (T0.5), 1.5 h (T1.5), and 2.5 h (T2.5) after the onset of phosphate limitation. For each time point, the band on the left is from wild-type strain 168, and the band on the right is from strain LS088. The PhoP and PhoR bands are indicated by arrows. Twenty micrograms of total protein was loaded in each lane. (D) The phoP (top panel) and phoR (bottom panel) transcript levels in cells of wild-type strain 168 (dark gray bars) and strain LSB088 (containing mutated PhoP binding boxes at the 3′ end of the phoPR operon) (light gray bars) growing in LPDM were measured by RT-qPCR. The level of each transcript was normalized to the level of transcript for the wild-type (WT) sample at time point T−1.5, which was assigned a value of 1.