Figure 1.
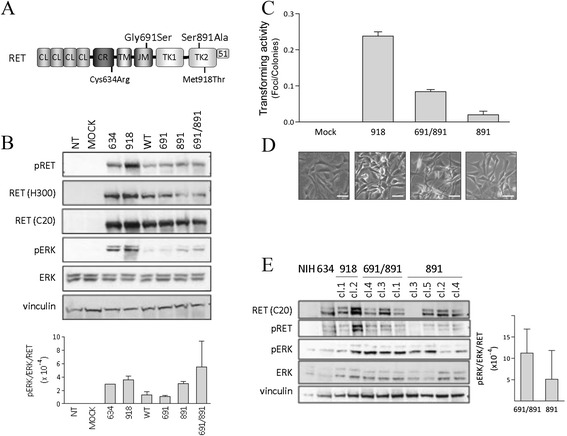
RET-S891A and RET -G691S/S891A mutants biochemical characterization and transforming activity. A. RET protein domains (CL, cadherin-like; CR, Cysteine-rich; TM, transmembrane; JM, juxtamembrane; TK, tyrosine kinase) and the RET51 isoform C-terminal tail are indicated. B. Western blot analysis of RET protein and associated ERK signaling in HEK293T cells transiently transfected. RET was detected by the antibody RET H300, specific for the RET extracellular portion, and by RET C20, specific for the intracellular portion of the RET long isoform. Vinculin is shown as protein loading control. Protein levels were quantified by densitometric analysis by Quantity One 4.6.6 software. Data are shown as mean expression value ± SD for 2 independent transfections. C. RET-S891A and RET-G691S/S891A mutants transforming activity by focus formation assay in NIH3T3 cells. pCDNA3 empty vector (Mock) and RET-M918T are included as negative and positive controls, respectively. The transforming activity, calculated as foci number/colonies number ratio, is reported as mean ± SD of triplicate counts. Data from one representative of two independent transfections are shown. D. Representative images of foci-derived clones. Scale bar 50um. E. Western blot analysis of RET protein and associated ERK signaling in NIH3T3 cells stably expressing the indicated RET variants: 634, RET-C634R; 918, RET-M918T; 691/891, RET-G691S/S891A; 891, RET-S891A. NIH, NIH3T3 untransfected cells. The partially and fully glycosylated forms of RET are identified in NIH3T3 cells. Vinculin is shown as protein loading control. Three RET-G691S/S891A clones and four RET-S891A clones were analysed. The ratio pERK/ERK/RET was calculated for each clone and the mean among clones ± SD was reported.
