Fig. 2.
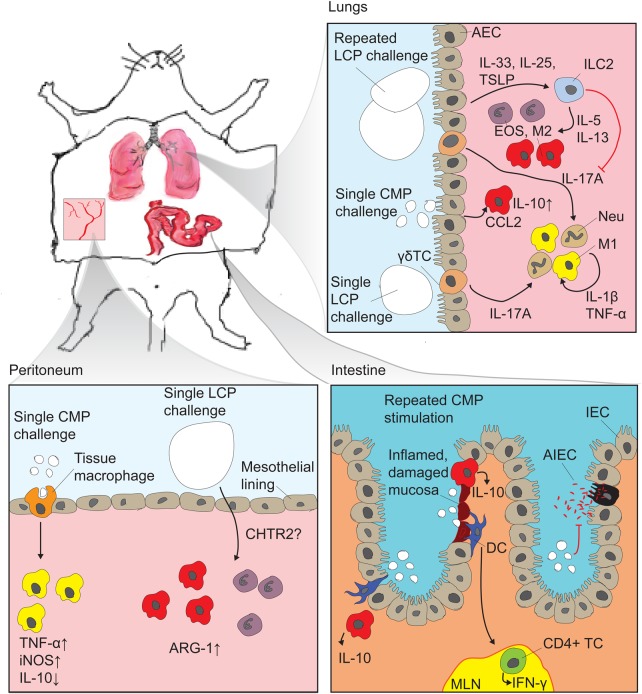
Murine in vivo observations of chitin immune-stimulating effects: The experimental approaches have generally been based on delivering chitin particles in liquid suspensions by one of the following routes: (i) intranasal or intratracheal delivery followed by broncheoalveolar lavage (BAL) analysis and histological examinations of lung tissue, (ii) IP injection followed by analysis of peritoneal lavage cell composition or (iii) gastrointestinal delivery by oral gavage followed by histological examination of mucosal barrier integrity and inflammation. In the lung, recent studies established a profound impact of epithelial derived signals, which hitherto have not been studied in other tissues. Repeated delivery with large chitin particles (LCPs) well above phagocytosable size induced IL-25, IL-33 and TSLP, which, via innate ILC2, induced type 2 innate immune responses characterized by tissue eosinophilia and alternative macrophage activation (M2). At the same time, ILC2s exerted an inhibiting effect on type 1 responses which would otherwise be driven by IL-17A released from γδ T cells (Van Dyken et al. 2014). Airway epithelial cells, upon binding of CMP, release CCL2 which further drive M2 (Roy et al. 2012), and CMP was observed to induce the production of IL-10 in BAL macrophages (Da Silva et al. 2009). Single exposure to LCPs leads to an IL-17A driven type 1 immune response which is strongly dependent on TLR-2. It is not known which cells are responsible for perceiving the chitin particles (Da Silva et al. 2008, 2009). In the peritoneum single exposure to CMP leads to phagocytosis-dependent proinflammatory signaling characterized by induction of TNF-α and inducible nitric oxide synthase while downregulating IL-10 expression. LCP induces tissue eosinophilia and M2 characterized by arginase 1 induction by a signaling pathway affected by CHTR2 (Kogiso et al. 2011). The possible signaling influence from the mesothelial cells lining the peritoneal cavity was not investigated. In murine colitis models, CMPs stimulated the accumulation of IL-10 producing cells, presumably alternatively activated macrophages, both at inflamed and non-inflamed sites. They have been shown in vitro to be internalized by dendritic cells which are then thought to stimulate IFN-γ production from CD4+ T cells (CD4+TCs) in the mesenteric lymph nodes (MLN) (Nagatani et al. 2012). Furthermore, CMPs inhibited adherence and invasion of adherent invasive E. coli in IECs (Kawada et al. 2008).