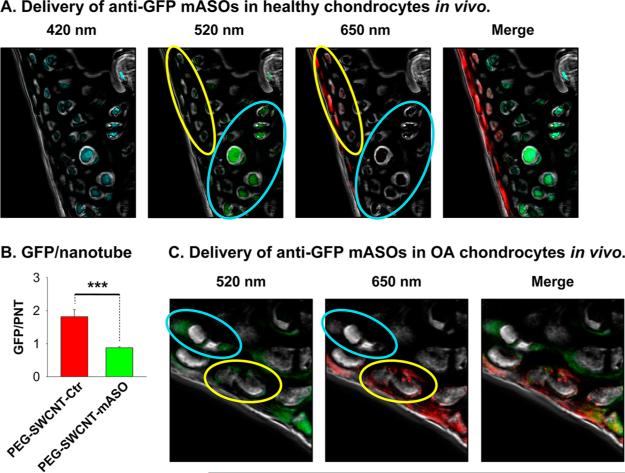
Figure 5.
(A, B) Delivery of anti-GFP mASOs by IA-injected PEG-SWCNTs in healthy chondrocytes in Vivo. Healthy (N = 3) female C57BL/6-Tg(UBC-GFP)30Scha/J mice were unilaterally IA-treated in the knee with 5 μg of PEG-SWCNT-mASOs in 10 μL of PBS. Contralateral knees were treated with PEG-SWCNT-Ctr. After 3 days, mice were sacrificed and cartilage cryosections prepared. Confocal images showed that nanotube-positive chondrocytes (650 nm signal) of IA-PEG-SWCNT-mASO-treated mice had faint GFP fluorescence (i.e., cells inside yellow oval), whereas nanotube-negative cells (i.e., cells inside cyan oval) had bright GFP signal. In contrast, both nanotube-positive and -negative cells of IA-PEG-SWCNT-Ctr-treated mice had bright GFP signal (see Figure S6C, Supporting Information). (B) GFP and PEG-SWCNT-650 signals were quantitated for nanotube-positive cells in four microscopic fields recorded for each of the six cartilage sections collected for IA-PEG-SWCNT-mASO- and IA-PEGSWCNT-Ctr-treated knees, and the GFP/nanotube signal ratio calculated. Statistics: nonparametric Mann–Whitney U analysis (***p < 0.001). (C) Delivery of anti-GFP mASOs by IA-injected PEG-SWCNTs in OA chondrocytes in Vivo. Four-month-old female C57BL/6-Tg(UBC-GFP)30Scha/J mice with OA (2 months post-DMM surgery) were injected in the OA knee with 5 μg of PEGSWCNT-mASOs in 10 μL of PBS. After 3 days mice, were sacrificed and cartilage cryosections prepared. Confocal images showed that nanotube-positive chondrocytes (650 nm signal) had faint GFP fluorescence (i.e., cells inside yellow oval), whereas nanotube-negative cells (i.e., cells inside cyan oval) had bright GFP signal.